Abstract
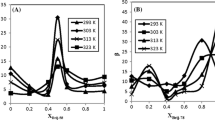
We investigate the linear and nonlinear optical property of Morin (MN) at different concentration (1 × 10–6 and 5 × 10–6 M) within AOT reversed micelle prepared by water-in-decane microemulsion having a constant molar ratio of water-to-surfactant molecules of 40 (W = [H2O]/[AOT] = 40) as well as the function of mass fraction of nano-droplet (MFD) values of 0.01,0.04, 0.07, and 0.1 by using UV-Visible, Fluorescence, FTIR, and Z-scan techniques. The steady-state measurement indicates that the presence of microenvironment can greatly affect the tautomeric structure of morin and also Morin property in microenvironment depends upon the amount of oil and Morin concentration. The increase in dipole moment from the ground state to excited state in microenvironment indicate the change in the molecular structure on morin. Morin does not show any nonlinear absorption property but the nonlinear refractive index is observed as a function of Morin concentration as well as MFD values which are due to the thermal agitation of formed dimers.
Graphical abstract

Morin nonlinearity
















Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
Sreedharan V, Venkatachalam KK, Namasivayam N (2009) Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis, invest. New Drugs 27:21–26
Höfener S, Kooijman PC, Groen J, Ariese F, Visscher L (2013) Fluorescence behavior of (selected) flavonols: a combined experimental and computational study. Phys Chem Chem Phys 15:12572–12581
Dhanasekar C, Rasool M, Eur M (2016) A dietary bioflavonol suppresses monosodium urate crystal-induced inflammation in an animal model of acute gouty arthritis with reference to NLRP3 inflammasome, hypo-xanthine phospho-ribosyl transferase, and inflammatory mediators. J Pharmacol 786:116–127
Ricardo KFS, Oliveira TTD, Nagem TJ, Pinto ADS, Oliveira MGA, Soares JF (2001) Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz Arch Biol Technol 44:263–267
Rattanachaikunsopon P, Phumkhachorn P (2010) Contents and antibacterial activity of flavonoids extracted from leaves of Psid guajava. J Med Plants Res 4:393–396
Kuzu M, Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Turk E (2018) Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed Pharmacother 106:443–453
Lee KM, Lee Y, Chun HJ, Kim AH, Kim JY, Lee JY, Ishigami A, Lee J (2016) Neuroprotective and anti-inflammatory effects of morin in a murine model of Parkinson's disease. J Neurosci Res 94:865–878
Zhang R, Kang KA, Piao MJ, Maeng YH, Lee KH, Chang WY, You HJ, Kim JS, Kang SS, Hyun J (2009) W Chem-Biol. Interact 177:21–27
Çelik H, Kucukler S, Çomaklı S, Özdemir S, Caglayan C, Yardım A, Kandemir FM (2020) Morin attenuates ifosfamide-induced neurotoxicity in rats via suppression of xidative stress, neuroinflammation and neuronal apoptosis. Neurotoxicology 76:126–137
Gottlieb M. et. al. (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia, Neurobiol Dis 23: 374–386
Lemkul JA, Bevan DR (2010) Destabilizing Alzheimer’s Aβ42 Protofibrils with Morin: mechanistic insights from molecular dynamics simulations. Biochemistry 49:3935–3946
Noor H, Cao P, Raleigh DP (2012) Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci 21:373–382
Caruana M, Högen T, Levin J, Hillmer A, Giese A, Vassallo N (2011) Inhibition and disaggregation of α-synuclein oligomers by natural polyphenolic compounds. FEBS Lett 585:1113–1117
Jangid AK, Pooja D, Kulhari H (2018) Determination of solubility, stability and degradation kinetics of morin hydrate in physiological solutions. RSC Adv 8:28836–28842
Abbad S, Wang C, Waddad AY, Lv H, Zhou J (2015) Preparation, in vitro and in vivo evaluation of polymeric nanoparticles based on hyaluronic acid-poly(butyl cyanoacrylate) and D-alpha-tocopheryl polyethylene glycol 1000 succinate for tumor-targeted delivery of morin hydrate. Int J Nanomedicine 10:305–320
Arriagada F, Correa O, Günther G, Nonell S, Mura F, Olea-Azar C, Morales J (2016) Morin flavonoid adsorbed on Mesoporous silica, a novel antioxidant nanomaterial. PLoS One 11:e0164507
Jangid AK, Agraval H, Gupta N, Yadav UC, Sistla R, Pooja D, Kulhari H (2019) Designing of fatty acid-surfactant conjugate based nanomicelles of morin hydrate for simultaneously enhancing anticancer activity and oral bioavailability. Colloids Surf B: Biointerfaces 175:202–211
Liu W, Guo R (2005) The interaction between morin and CTAB aggregates., J. Colloid Interface Sci 290:564–573
Waddad AY, Abbad S, Yu F, Munyendo WL, Wang J, Lv H, Zhou J (2013) Formulation, characterization and pharmacokinetics of Morin hydrate niosomes prepared from various non-ionic surfactants. Int J Pharm 456:446–458
Stagnoli S, Alderete LS, Luna MA, Agostini E, Falcone RD, Niebylski AM, Correa NM (2020) Catanionic nanocarriers as a potential vehicle for insulin delivery. Colloids Surf B: Biointerfaces 188:110759–110765
Sett R, Sen S, Paul BK, Guchhait N (2018) How does nanoconfinement within a reverse micelle influence the interaction of phenazinium-based photosensitizers with DNA? ACS Omega 3:1374–1377
Bozkurt E, Onganer Y (2018) Photophysical features of coumarin 120 in reverse micelles. J Mol Struct 1173:490–495
Pileni MP (1993) Reverse micelles as microreactors. J Phys Chem 97:6961–6973
Uskoković V, Drofenik M (2007) Reverse micelles: inert nano-reactors or physico-chemically active guides of the capped reactions. Adv Colloid Interf Sci 133:23–34
Trivedi R, Kompella UB (2010) Nanomicellar formulations for sustained drug delivery: strategies and underlying principles. Nanomedicine. 5:485–505
Naoe K, Yoshimoto S, Naito N, Kawagoe M, Imai M (2011) Preparation of protein nanoparticles using AOT reverse micelles. Biochemical Engineering J 55:140–143
Rahdar A, Aliahmad M, Kor AM, Sahoo D (2019) Probing the reverse micelle environment with a cationic dye by varying oil and water content of micelles, Spectrochim. Acta, Part A 210:165–170
Chatzidaki MD, Papavasileiou KD, Papadopoulos MG, Xenakis A (2017) Reverse micelles as antioxidant carriers: an experimental and molecular dynamics study. Langmuir 33:5077
Rahdar A, Bagheri H (2019) An insight into the effect of nano-confinement on some of photo-physical parameters of dye. Appl Phys A Mater Sci Process 125:648
Jian JT, Zhiguo S, Guang CY (2009) The nonlinear optical response of a fluorine-containing azoic dye. Opt Commu 283:1110–1113
He T, Wang C, Study on the nonlinear (2008) Optical properties of three azo dyes by Zscan measurements. J Mod Opt 55:3013–3017
Raikar U, Renuka C, Nadaf YF, Mulimani B, Karguppikar A (2006) Soudagar M solvent effects on the absorption and fluorescence spectra of coumarins 6 and 7 molecules: determination of ground and excited state dipole momentSpectrochim. Acta, Part A 65:673–677
Panhwar QK, Memon S (2014) Synthesis of Cr(III)-Morin complex: characterization and antioxidant study. Sci World J 2:845208–845216
Nafisi S, Hashemi M, Rajabi M, Ali Tajmir-Riahi H (2008) DNA adducts with antioxidant flavonoids: Morin, Apigenin, and Naringin. DNA Cell Biol 27:433–442
Owrutskya JC, Pomfret MB, Barton DJ, Kidwell DA (2008) Fourier transform infrared spectroscopy of azide and cyanate ion pairs in AOT reverse micelles. J Chem Phys 129:024513–024523
Guowei Z, Ganzuo L, Wenjun C, Anjing L, Meng B (2002) FT-IR studies on the conformation and effective head-group area of AOT molecules in W/O microemulsions. Science in China Series B: Chemistry 45:68–72
Bark KM, Im SE, Seo JJ, Park OH, Park CH, Park HR (2015) Spectroscopic study on the stability of Morin in aqueous solution. Bull Kor Chem Soc 36:498–502
Liu R, Yang J, Wu X, Hua S, Sun C (2001) Interaction of morin with CTMAB: aggregation and location in micellar. Spectrochim Acta A 57:2561–2566
Valeur B, Berberan-Santos MN (2012) Molecular fluorescence, principles and Applications,2 ed. Wiley-VCH, Weinheim
Rahdar A, Almasi-Kashi M, Mohamed N (2016) Light scattering and optic studies of Rhodamine B-comprising cylindrical-like AOT reversed micelles. J Mol Liq 223:1264–1269
Höfener S, Kooijman PC, Groen J, Ariese F, Visscher L (2013) Fluorescence behavior of (selected) flavonols: a combined experimental and computational study. Phys Chem Chem Phys 15:12572–12576
Sheik-Bahae M, Said AA, Wei TH, Hagan DJ, Stryland V (1990) Sensitive measurement of optical nonlinearities using a single beam IEEE J. Quantum Electron 26:760–769
Sheik-Bahae M, Said AA, Stryland V (1989) High-sensitivity, single-beam n2 measurements. Opt Lett 14:955–957
Acknowledgements
Authors gratefully acknowledge the financial support for this work from the University of Zabol (grant number. UOZ-GR-9618-143). D.S. would like to thank UGC for his UGC Assistant Professorship.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Abbas Rahdar], [Esmael Sanchooli], [R. Karimzadehc] and [Dibakar Sahoo]. The first draft of the manuscript was written by [Dibakar Sahoo], [Abbas Rahdar] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
The optical property of Morin can greatly be affected by the microenvironment.
The tautomeric form of MN can also be affected by its concentration and oil content of AOT
The increase in dipole moment from the ground state to excited state in microenvironment.
Morin does not show any nonlinear absorption property.
Morin shows the nonlinear refractive index as a function of Morin concentration as well as MFD values.
Supplementary Information
ESM 1
(DOCX 37 kb)
Rights and permissions
About this article
Cite this article
Rahdar, A., Sanchooli, E., Karimzadeh, R. et al. Investigation on the Linear and Nonlinear Properties of Morin in Presence of Reverse Micelle and Different Oil Content in Reverse Micelle. J Fluoresc 31, 373–383 (2021). https://doi.org/10.1007/s10895-020-02665-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-020-02665-1