Abstract
In this work, a series of linear and star-shaped poly(ε-caprolactone)s (SPCL) with various arm numbers were successfully synthesized with ring-opening polymerization (ROP) with the initiators having different number of hydroxyl functional groups. The molecular characteristics of synthesized PCLs were analyzed via Fourier Transform Infrared Spectroscopy (FTIR), proton nuclear magnetic resonance spectra (1H NMR), thermogravimetric analyzer (TGA), differential scanning calorimetry (DSC) and gel permeation chromatography (GPC) measurements. Then PCLs were melted with poly(lactic acid) (PLA) by utilizing a micro-compounder at a constant blending ratio (90/10% weight) and constant 1,4-phenylene diisocyanate (PDI) (1% weight) as a commercial compatibilizer. It was reported that adding SPCL improved mechanical properties of PLA. The three-armed star shaped PCL (3SPCL) caused significant decrease in modulus due to its high molecular chain mobility when compared linear, four- and six-armed PCLs. An increment was observed in the elongation at break values with incorporation of star polymers and it increased from 4 to 9%. Scanning electron microscopy (SEM) photos indicated that immiscibility of the two biodegradable polymers were enhanced and thus mechanical improvement were achieved.
Similar content being viewed by others
Introduction
Biocompatible polymers have attracted good attention in the rapid growth of plastic industry obtained from the non-chemical resources, which could be a good choice to the limited fossil resources and environmental concerns. The most favored biodegradable polymers are aliphatic thermoplastic polyesters like PLA, PCL, polyhydroxy butyrate (PHB), and poly(butylene adipate terephthalate) (PBAT). PLA is the most important linear polyester that are generally manufactured from renewable feedstocks, like wheat, corn, potato, and sugar [1,2,3]. Owing to its mechanical characters, processability and biodegradability, PLA is widely utilized in industrial fields [3, 4]. It has attractive physical properties, including a good optical clarity, high strength, modulus and melting temperature (Tm). However, pure PLA polymer has some serious drawbacks for industrial applications like having a low toughness, brittleness, poor melt strength, poor long-term performance, low heat bending temperature, low thermal resistance, narrow processing window and limited gas barrier properties. Especially, to improve its brittleness and low toughness properties have been attracting the attention of a lot of researchers to widen the application of PLA. So, numerous procedures like copolymerization, plasticization, blending with other suitable ductile polymers, and composites with rigid additives, have been suggested to develop properties like toughness of PLA [5,6,7,8,9,10].
One of the convenient approaches to get over these restrictions to employ physical blending with another polymer. In other words, the physical blending of brittle polymer with ductile polymer has been extensively used is to enhance the fracture toughness and impact resistance of the base polymer [11, 12]. PCL as a ductile polymer has been extensively utilized for blending with PLA to improve the toughness, owing to its biodegradability, biocompatibility, low glass-transition temperature (Tg), flexibility, high toughness etc. It was detected that the blends of PLA with biodegradable PCL have better mechanical properties than neat PLA [11, 13,14,15,16,17].
The miscibility, viscoelastic, thermodynamic and mechanical behaviors of the PLA/PCL blend system have been extensively studied [12, 18,19,20]. These blends were prepared at different ratios and molecular weight of either components to improve desired properties [18, 21, 22]. Yeh et al. prepared PLA/PCL blend with different mass ratios via melt blending to examine morphology, miscibility, mechanical, crystallization and thermal behavior. It was showed that two polymers were partial miscible, when PCL amount increased in the PLA phase, a reduction on the Tg value was found [21]. Semba and coworkers showed the influence of crosslinking on mechanical behavior of PLA/PCL blends via utilizing dicumyl peroxide (DCP) like a compatibilizer. It was observed that improving interfacial adhesion caused increase in the impact and tensile brittle surface by adding DCP. Also, DCP was determined to be a good compatibilizer due to the high tensile strain value at poor DCP concentration for PLA/PCL blend systems [23]. Rao and coworkers examined rheological and mechanical behaviors of PLA/PCL blend system and found that the fracture character of PLA turned more ductile and viscosity property of the PLA/PCL blend also enhanced via addition of PCL [24]. However, it was found that PCL and PLA are not miscible polymers and observed a phase separation between them [12, 25,26,27]. Broz and coworkers prepared a series of PLA/PCL blends via various mass fraction to examine the mechanical and structural behaviors of the blends. They found that there were minimum interactions and a small adherence at the interface of PLA/PCL when PCL formed the continuous matrix [22]. Navarro-Baena and coworkers studied the shape memory feature of PLA/PCL composites in various concentrations. It was showed that only these blend system with PCL at a ratio of 30 wt % display shape memory feature [28].
Recently, star-shaped polymers having unique three-dimensional architectures have attracted growing focus of scientists outstanding to some properties like mechanics, rheology, and easy control of surface functionality [29, 30]. Star-shaped polymers have lower Tg and melt viscosity, lower crystalline, smaller hydrodynamic radius owing to their compact in size and high intensity of end-functional groups compared with linear ones having similar molecular weights [31, 32]. A series of star-shaped PCLs were synthesized with various number of arms according to core structure and different arm lengths in the literature [33,34,35,36,37]. Besides, there are several studies in the literature that prepared blends of PLA with synthesized star-shaped PCL. Qin and coworkers prepared a series of blends of PLLA with star-shaped PCL-PLA copolymer at different contents. They determined that the mechanical behavior of PLLA were markedly improved with enhancing star-shaped PCL content [30]. Li et al. produced a series of star-shaped PCLs with dissimilar molecular weight and mixed with PLA to obtain blended fiber membranes via electrospinning technique. They examined the influence of multiple-armed structure on the mechanics and thermodynamics feature of the electrospinned fiber membranes [32]. Deokar et al. reported multiarmed star-branched PCL-PLA polymers to enhance the toughness of PLA. They determined that the elongation of the PCL-PLA/PLA blend enhanced with the number of arms of the multiarmed star-branched polymers [31]. In previous study, we synthesized star-shaped PCLs (SPCLs) with POSS core and prepared melt-blend with commercial PLA by utilizing twin mini screw extruder to increase toughness of PLA. The mechanical, morphological, and thermal behaviors of these blends were examined as a function of star-shaped polymer type possessing different molecular weight depending on different chain lengths at constant PLA/SPCL ratio. It was found that an increment was observed on toughness of commercial PLA due to relatively low Tg of ductile PCL. Furthermore, addition of SPCL polymers into commercial PLA improved morphological and mechanical properties [38].
In here, synthesis and characterization of linear and star-shaped PCLs with various numbers of arms (LPCL and SPCLs, respectively) were reported and the effects of multi-armed star structure rather than linear ones on the toughness and some characteristics of PLA were investigated. These polymers were produced with the ROP of ε-CL utilizing core-first method beginning from multisite initiators with two, three, four, and six hydroxyl functionalities (Scheme 1). The commercial PLA and synthesized PCL polymers were compounded by using mini extruder and next injection molding. PDI was utilized to enhance the compatibility of blends of these polymers. PDI can stabilize the morphology since it can give reaction with -COOH and -OH terminate groups of two polymers, PLA and PCLs [38,39,40].
Experimental
Materials
The monomer ε-caprolactone (ε-CL, Aldrich, 97%) was dried over CaH2, distilled under reduced pressure prior to utilize and kept under nitrogen. PLA (PLI005, melt flow index: 10–30 g/10 min at 190 °C/2.16 kg, NaturePlast), ethylene glycol (99.8%, Sigma–Aldrich), methanol (CH3OH, Sigma-Aldrich, ≥ 99.8%), pentaerythritol (≥ 99%, Aldrich), dipentaerythritol (for synthesis, Sigma-Aldrich), 1,1,1-tris(hydroxymethyl)propane (98%, Aldrich), 1,4-phenylene diisocyanate (PDI, Aldrich), tin(II) 2-ethylhexanoate (Sn(Oct)2, 95%, Aldrich), dichloromethane (DCM, 99.8%, Sigma–Aldrich) were purchased and used without further purification.
Preparing of PCLs having various numbers of arms
Due to the similar reaction conditions, only the synthesis procedure of the three-armed star-shaped PCL (3SPCL) was given as the representative example (see the Supporting Information for the experimental details of other PCLs). 3SPCL synthesized via ring opening polymerization (ROP) of ε-CL monomers in bulk utilizing a catalyzer (Sn(Oct)2) with a procedure given in elsewhere [29, 34, 41, 42]. ε-CL (20.42 g, 178.878 mmol), 1,1,1-tris(hydroxymethyl)propane (0.40 g, 2.981 mmol), and Sn(Oct)2 (0.36 g, 0.894 mmol) were added one-by-one to a fire dried polymerization flask. The reaction mixture deoxygenated via flushing with inert argon atmosphere for 15 min, the flask was closely sealed, placed in an oil bath set at 115 °C and stirred for one night. Then, the polymerization flask was cooled to 25 oC the mixture was diluted in DCM (≈10 mL), then precipitated in cold methanol. 3SPCL was filtered by using a G4 filter and kept under vacuo until drying.
Yield: 19.90 g (95.6%). FT-IR (cm−1): 3470 (O–H, broad); 2941 (C-H); 2868 (C-H); 1721 (C = O); 1471 (C-H); 1240 ((C = O)-O); 1045 (C–O–C). 1H NMR (CDCl3, δ, ppm): 4.08 (m, -O(C = O)CH2CH2CH2CH2CH2O(C = O) in PCL); 4.03 (s, CH3CH2C(CH2O(C = O))3); 3.67 (m, terminal -CH2OH in PCL); 2.32 (m, -O(C = O)CH2CH2CH2CH2CH2O(C = O) in PCL); 1.66 (m, -O(C = O)CH2CH2CH2CH2CH2O(C = O) in PCL, and CH3CH2C(CH2O(C = O))3); 1.40 (m, -O(C = O)CH2CH2CH2CH2CH2O(C = O) in PCL); 0.89 (t, CH3CH2C(CH2O(C = O))3).
Blend preparation
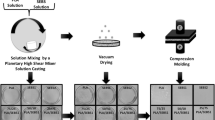
PLA was hold at 65 °C for 24 h with vacuum before the experiments. SPCL and PLA polymers were melt-blended with Xplore Instruments 15 ml twin-screw mini extruder (The Netherlands). The mixing of two polymers were performed at 190 °C and 2 min mixing time, the screw speed of the extruder was 100 rpm, following injection molding machine (Xplore 12 ml) was used to produce mechanical test specimens. The temperatures for mold and melt were 25 °C and 190 °C. The blends were prepared at a range of 90/10 and PDI was added as 1% by weight.
Characterization of synthesized polymers and blends
1H NMR (Varian INOVA Unity, 500 MHz) and FTIR spectroscopy (ATR Bruker-Tensor 27 spectrometer) were utilized for characterization of synthesized polymers. ATR-FTIR spectra of all intermediate and star polymers were performed in the frequency range of 600–4000 cm−1 at 25ºC via using the attenuated total reflectance (ATR) technique. GPC analysis of polymers were carried out on Viscotek RI max system comprising of an oven, a pump, an autosampler, two LT4000L Viscotek T-columns (7 μm particle size, 1500 A pore size, 300 mm length, 8 mm i.d.), and a Viscotek refractive index detector (VE3580). THF was utilized as the eluent at a flow rate of 1.0 mL/min at 23 °C. Linear PS-based calibration curve was used to calculate GPC results via Viscotek OmniSEC software. Thermal behavior of the obtained polymers and blends were detected on a Mettler Toledo DSC 1 Star instrument DSC analyzer under N2 flow from 25 to 250 °C at a heating/cooling rate of 10 °C/min. All of the experiments were reported as an average of at least three results. Mettler Toledo TGA 1 Star System was utilized for thermal properties of polymers, the temperature was between room temperature and 650 °C with 10 °C min−1 heating rate. The melt flow behaviors of blends were determined by vertical force measurements which were recorded from micro-compounder during feeding. The detail information for vertical force measurements can be found from previous report [38]. Mechanical tests of the PLA/SPCL blends were performed with an Instron universal testing machine (Model 3345) at room temperature. The dog-bone shaped test specimens which were used for tensile tests were produced according to ISO 527 standard. ZWICK/ROELL impact testing machine was used to determine impact strength and Izod impact test bars were produced ISO 180 standard. The morphological properties of prepared blends were analyzed via Field Emission Scanning Electron Microscope (SEM, FEI- QUANTA FEG 250).
Results and discussion
Preparation of LPCL and SPCL polymers
The LPCL and SPCL polymers were produced via ROP by using the multisite initiators having varied numbers of hydroxyl groups (see Scheme 1). Ethylene glycol, 1,1,1- tris(hydroxymethyl)propane, pentaerythritol, and dipentaerythritol were utilized as the multisite initiators to obtain two-, three-, four-, and six-armed PCLs in turn.
The chemical characters of the obtained PCLs having different number of arms were clearly confirmed via 1H NMR and FTIR analysis methods and depicted in Fig. 1 and Fig. S1, respectively. All the PCL polymers with the same end-functional units and ε-CL repeating units except for their initiator residues indicated the same signals according to both spectra. As seen from Fig. S1, hydroxyl end-functional PCLs display the broad absorption corresponding to the hydroxyl stretching at 3470 cm−1. The intensity of -OH peak in the spectra of PCLs were reduced as the Mw of the polymer enhances, exhibiting that ROP of ε-CL proceeds through hydroxyl groups (marked with blue column in Fig. S1). Besides, the sharp and strong signal at 1721 cm−1 is ascribed to the C = O group in the repeating units of the polymer chains.
Regarding the 1H NMR spectra, the methylene group signals of the repeating parts of LPCL were obtained at 1.39 (He), 1.65 (Hc), 2.30 (Hb), and 4.06 (He) ppm (Fig. 1a). In addition, the protons in terminal methylene groups (Hf) and ethylene glycol initiator residue (Ha) exhibited resonances at 3.65 and 4.11 ppm in turn. The methylene proton signals of the ε-CL repeating parts and the terminal group of star-shaped PCLs were found to be almost identical to those of LPCL. In addition, the proton peaks belong to 1,1,1- tris(hydroxymethyl)propane, pentaerythritol, and dipentaerythritol as the multisite initiators observed at 0.89 (Ha), 1.66 (Hb) and 4.03 (Hc) ppm in Fig. 1b, at 4.13 (Ha) ppm in Fig. 1c, 3.57 (Ha) and at 3.41 (Hb) ppm in Fig. 1d in turn. 13C-NMR spectra are given Supporting Information Fig. S2.
The number‐average molar weights (Mn) of PCLs along with molecular weight distributions (Mw/Mn) data were recorded by GPC chromatograms (see Supporting Information Fig. S3) and the associated data are given in Table 1. Both of the PCLs showed symmetrical unimodal peaks and had low Mw/Mn values (1.24–1.33) demonstrating that the purified ROP yields included only the desired PCLs. The theoretical molecular weight (Mn,theo) of PCLs was calculated from the feed ratio and yield. Also, the number average molecular weights (Mn,NMR) of the PCLs were calculated by comparing the integral area ratio between the signals of any methylene protons of the ε-CL repeating units of PCL main chains and that of (Hh) methylene protons at the termini of PCL chains at 3.65 ppm from their 1H NMR spectra (Table 1). The experimental (Mn,GPC) and theoretical (Mn,theo) molar weight values were obtained close to each other indicating good control over the molecular distribution. The Mw/Mn of star PCL polymers was lower when compared to linear PCL, but values increased with increasing molecular weight due to the number of arms in star polymers.
The thermal properties of obtained polymers examined via TGA and DSC measurements (see the Supporting Information Figs. S4 and S5). The maximum weight loss (Td,max), onset decomposition (Td,onset) temperatures and char yields of PCLs significantly increased with enhancing molecular weight of these polymers (Table 1). DSC thermograms of PCLs (the Supporting Information Fig. S5) was used to find thermal transitions of the produced PCLs with various arm numbers and given in Table 1. DSC data revealed that Tm2 temperature of PCLs in the second heating run decreased with increasing number of arms of these polymers. It is difficult to define Tg values of PCL polymers with DSC analysis since the Tg of PCL is around -60 \(^\circ\)C. Therefore, Tg values of these polymers could not be reported in Table 1. The crystallinity of the synthesized PCL polymers (Xc2) was also determined from DSC measurements by utilizing the melting enthalpy (ΔHm0) as 139.6 J/g for completely crystalline PCL [37, 42, 43]. The Xc of LPCL has maximum value as 65.3% and a decrease was observed with the increment of the arm number of SPCLs. The crystallinity order is as follows: Xc,LPCL > Xc,3SPCL > Xc,4SPCL > Xc,6SPCL. This result is in concordance with literature and a similar trend is reported by Wang and Dong [37].
Vertical force (VF) measurements
The vertical force (N) is utilized for comparing the melt viscosity of polymer mixtures basically it cannot be used exactly in units of viscosity. It is measured by extrusion process and detailed information is given previous report [38]. The change of VF in PLA/SPCL and PLA/PDI/SPCL blends is given in Fig. 2. The melt viscosity of pure PLA has a maximum value in comparison to star PCL blends. It is clear that melt viscosity of blends has lower values with the increment of arm number of the SPCL. These polymers generally have smaller solution and melt viscosity and less polymer entanglement in comparison to linear ones [43]. So, inclusion of SPCLs to PLA matrix causes easier blending with a reduction in vertical force values [31]. This also supports that star PCL behaves like a plasticizer and gets enhancement of properties of PLA [21, 38, 44]
The VF variation of blends including PDI is given in Fig. 2b. There is a little increment in the melt viscosity due to the chain extender role of PDI. PDI acts a compatibilizer and bonds the chain ends of PLA and SPCL by forming the linear PLA, SPCL and PLA-co-SPCL [39, 45, 46]
Mechanical properties of PLA/SPCL blends
The mechanical characteristics of the binary blends usually exist between the properties of the two components. PLA is described as a brittle biopolymer with high yield strength, tensile modulus, and low elongation value. When blended with linear PCL polymers it enhances the mechanical properties due to the ductile structure of PCL. The elastic modulus generally decreases and elongation values increases with the inclusion of flexible PCL polymers [18, 38]. The variation of the tensile modulus of the blends with PLA and SPCL are represented in Fig. 3 with the result of LPCL blends. It was determined that the toughness of the system was improved simultaneously after blending with SPCLs. There is a decrease with the addition of SPCL polymers as expected from literature since PCL polymers characteristically have less modulus and tensile strength in comparison to PLA [18, 47, 48, 49]
The variation of elongation (%) with addition of SPCL polymer to PLA with and without PDI is given in Fig. 4. It is distinctly seen that single PLA has lowest value due to its brittle nature. The addition of SPCL polymers incorporates elasticity to the blend system due to flexible PCL arms and an increment is observed in the elongation (%) values with the increase in arm number of these polymers. It is determined that fragile nature of PLA turns into ductile with incorporation of SPCL polymers. Deokar et al. found similar trend by blending PLA with star-shaped of LA-block-CL copolymers [31].
The variation of yield strength of blends is represented in Fig. 5. It is seen that yield strength increases with the addition of LPCL polymer, [38] but there is no significant change with the addition of SPCL polymers with different arm numbers. PDI also has no effect on yield strength values of blends.
The change of the Izod impact strength of blends is depicted in Fig. 6. There is a tremendous increment with the addition of SPCL polymers because of their crack-pinning effect and with the increment of arm number of the SPCL in parallel to elongation at break values. Besides, PDI addition to blend system is caused also an increment in impact strength when compared to pure PLA, but a decrement was observed in comparison with pure PLA/SPCL blends.
One of the frequently used methods for determining the physical properties of materials is hardness measurement. Generally, hardness can be expressed as resistance against friction, scratching, cutting or plastic deformation under static or dynamic loading conditions. Unlike other tests, the results obtained are used for comparison purposes rather than being used directly. The hardness of PLA and SPCL blends are given in Fig. 7. There is a slight increase with LPCL blends, but the hardness is almost same with SPCL polymers. PDI has an insignificant impact on hardness.
Thermal properties of PLA/SPCL blends
Thermal properties of PLA/SPCL and PLA/PDI/SPCL blends, which were observed from TGA and DSC analysis, were given in Table 2. The test samples were prepared by cutting the fractured tensile test bars from the center. DSC plots representing the second heating of blends were given in Fig. 8. The Tg value of pure PLA is 55.4 \(^\circ\)C and it is nearly equal to the melting temperature of synthesized SPCLs given in Table 1 (55.31–52.48 \(^\circ\)C). The blending PLA with the different armed star polymers resulted with a slight decrease in Tg from 55 to 51 \(^\circ\)C. This result is consistent with Tg value of 50.3 \(^\circ\)C for eight-armed SPCL blends reported in the previous paper due to the plasticization behavior of star-shaped PCL polymers compared to neat PLA [38]. There is no substantial effect of PDI on Tg value of these blends. Also, it was observed that an increase in the Tm values of blends owing to increase of the arm number of SPCL. Besides, addition of SPCL to PLA affected the cold crystallization temperatures (Tc) and there is a decrease in Tc values in comparison to single PLA due to the behavior of SPCL as nucleation agent.
The crystallinity (Xc) is important in the mechanical behaviors of binary blends and is calculated for PLA from the following equation by considering the quantity of PLA in the blends:
where \(\Delta {H}_{m}\) is the melting enthalpy (J/g), \(\Delta {H}_{C}\) is crystallization enthalpy (J/g) of the samples and \(\Delta {H}_{m}^{0}\) is the standard melting enthalpy of PLA taken to be 93.1 J/g [1, 44, 46, 49, 50, 51]. \(\varphi\) is the weight fraction of PLA in the blends. Pure PLA has a crystalline value of 0.2%, indicating that the crystals formed during cold crystallization (Table 2). In general, the crystallinity values of the blends are below 7%. The incorporation of SPCL results in an increment in the crystallinity of PLA and it increases with the increase of the arm number of the SPCLs. The dispersed PCL phases inside the PLA matrix behaves as nucleating points showing SPCLs have compatibilizing effect and slightly enhances crystallization of PLA [18]. It is determined that such an interface system can act like a plasticizer during the crystallization process of PLA [30]. It is known that addition of plasticizers, here SPCLs, lowers the modulus and increases the impact strength values as given in Fig. 3 and 6. The enhancement of the mechanical characters can be ascribed to developed crystallinity of PLA in the blends [18, 30, 38].
TGA plots of PLA/SPCL and PLA/PDI/SPCL blends are represented in Table 2 and Fig. 9. It can be said that the decomposition mechanism was no effected by addition SPCL polymers to neat PLA and all the blends show one stage degradation process, the differences is the degradation starts earlier in comparison to single PLA. The temperature at 5 wt% mass loss (T5%) of the single PLA was observed around 338 °C, and the decomposition of the blends started to at 289 and 285 °C with SPCL polymers. With the inclusion of PCL to the PLA, there is a general decrement in all temperatures (T5, T10, T90, and Tmax) due to ductile properties of PCLs. Generally, Tmax values of star polymers were found to be higher than linear PCL polymers with increasing number of arms. In addition, when PDI was added, it was determined that Tmax were smaller than those without PDI. Due to the chain extending the effect of PDI, the molecular weight increases with the enhance in the number of arms. Therefore, an increase in Tmax values was determined in the blends with PDI.
Morphology of blends
SEM was employed for morphological analysis of the blend system. Mechanical behavior of polymer blends is connected with well dispersed phase morphology. The fracture surface of single PLA and PLA/PDI with large cracks and flat plateau on it are represented in previous work [38]. SEM images of PLA/SPCL and PLA/PDI/SPCL specimens are displayed in Fig. 10. PLA matrix is continuous phase and PDI and the added SPCL polymers are dispersed phase in our multicomponent blend system4. Blending PLA with PCL polymers changes its fracture behavior [38]. Since PLA has large cracks on the fracture surface, addition of linear PCL causes smaller cracks with a nice distribution of globular PCL phase in PLA. The immiscibility is enhanced by incorporation of PDI to blend system due to the chain extension reactions. No apparent phase separation was observed in these figures except rough or slim crack patterns. Besides, the strong cracks disappear, and homogeneously dispersed SPCL phases begin to appear in the continuous PLA phase by the addition of SPCL polymers. The distribution of SPCL in PLA is more homogeneous and this shows SPCL is better miscible when compared to linear PCL (Fig. 10). Two phases cannot be seen with incorporation of PDI especially in 6SPCL blends, so it enhances the immiscibility, and the SEM results are coherent with the formation of a PLA-co-SPCL due to the reactive compatibilization.
Conclusion
A series of different arm number SPCL polymers were synthesized to develop the morphological and especially mechanical behavior of neat PLA. Linear, three-, four- and six-armed SPCLs were blended with PLA by using a mini extruder to compare the arm number effect of PCL. It was found that a good increase was observed in elongation (%) values with a decrease in modulus of PLA/SPCL blends rather than linear PCL polymers. Also, it was shown that the fragile nature of pure PLA turned to ductile by blending via SPCL polymers with different arm numbers. The impact properties of the blends were also considerably improved. It was observed in the lights of the SEM photos that immiscibility of the two polymers were enhanced and thus mechanical improvement were achieved. It can be concluded that using star- shaped PCL polymers rather than linear ones is more successful to improve mechanical behavior of neat PLA. Hence, the application field of PLA was broadened by blending with SPCL polymers, these blends can be utilized for various daily and industrial applications such as food packaging, disposable products, biomedical, etc.
Supporting Information
Synthesis procedure of two armed PCL (LPCL), four and six armed PCL (4SPCL, 6SPCL) and some of molecular characterization results of the polymers are given in the supplementary material provided for this article.
References
Kodal M, Sirin H, Ozkoc G et al (2019) Long-and short-term stability of plasticized poly (lactic acid): effects of plasticizers type on thermal, mechanical and morphological properties. Polym Bull 76(1):423–445
Tuna B, Ozkoc G et al (2017) Effects of diisocyanate and polymeric epoxidized chain extenders on the properties of recycled poly (lactic acid). J Polym Environ 25(4):983–993
García-Campo MJ, Boronat T, Quiles-Carrillo L, Balart R, Montanes N et al (2018) Manufacturing and characterization of toughened poly (lactic acid) (PLA) formulations by ternary blends with biopolyesters. Polymers 10(1):3
Drumright RE, Gruber PR, Henton DE et al (2000) Polylactic acid technology. Adv Mater 12(23):1841–1846
Liu Z, Hu D, Huang L, Li W, Tian J, Lu L, Zhou C et al (2018) Simultaneous improvement in toughness, strength and biocompatibility of poly (lactic acid) with polyhedral oligomeric silsesquioxane. Chem Eng J 346:649–661
Mauck SC, Wang S, Ding W, Rohde BJ, Fortune CK, Yang G, Ahn S-K, Robertson ML et al (2016) Biorenewable tough blends of polylactide and acrylated epoxidized soybean oil compatibilized by a polylactide star polymer. Macromolecules 49(5):1605–1615
Huang Y, Chang R, Han L, Shan G, Bao Y, Pan P et al (2016) ABA-type thermoplastic elastomers composed of poly (ε-caprolactone-co-δ-valerolactone) soft midblock and polymorphic poly (lactic acid) hard end blocks. ACS Sustainable Chem Eng 4(1):121–128
Ojijo V, Ray SS et al (2015) Super toughened biodegradable polylactide blends with non-linear copolymer interfacial architecture obtained via facile in-situ reactive compatibilization. Polymer 80:1–17
Raquez J-M, Habibi Y, Murariu M, Dubois P et al (2013) Polylactide (PLA)-based nanocomposites. Prog Polym Sci 38(10–11):1504–1542
Carvalho JR, Conde G, Antonioli ML, Dias PP, Vasconcelos RO, Taboga SR, Canola PA, Chinelatto MA, Pereira GT, Ferraz GC et al (2020) Biocompatibility and biodegradation of poly (lactic acid)(PLA) and an immiscible PLA/poly (ε-caprolactone)(PCL) blend compatibilized by poly (ε-caprolactone-b-tetrahydrofuran) implanted in horses. Polymer J 52:629–643
Ostafinska A, Fortelný I, Hodan J, Krejčíková S, Nevoralová M, Kredatusová J, Kruliš Z, Kotek J, Šlouf M et al (2017) Strong synergistic effects in PLA/PCL blends: Impact of PLA matrix viscosity. J Mech Behav Biomed Mater 69:229–241
López-Rodríguez N, López-Arraiza A, Meaurio E, Sarasua J et al (2006) Crystallization, morphology, and mechanical behavior of polylactide/poly (ε-caprolactone) blends. Polym Eng Sci 46(9):1299–1308
Takayama T, Todo M et al (2006) Improvement of impact fracture properties of PLA/PCL polymer blend due to LTI addition. J Mater Sci 41(15):4989–4992
Todo M, Harada A, Tsuji H et al (2007) Fracture characterizarion of biodegradable PLLA polymer blends. 16th International conference on composite materials, Kyoto 1–6
Wachirahuttapong S, Thongpin C, Sombatsompop N et al (2016) Effect of PCL and Compatibility Contents on the Morphology, Crystallization and Mechanical Properties of PLA/PCL Blends. Energy Procedia 89:198–206
Urquijo J, Guerrica-Echevarría G, Eguiazábal JI et al (2015) Melt processed PLA/PCL blends: Effect of processing method on phase structure, morphology, and mechanical properties. J Appl Polym Sci 132(41):42641–42649
Chen H, Yu X, Zhou W, Peng S, Zhao X et al (2018) Highly toughened polylactide (PLA) by reactive blending with novel polycaprolactone-based polyurethane (PCLU) blends. Polym Test 70:275–280
Simões C, Viana J, Cunha A et al (2009) Mechanical properties of poly (ε-caprolactone) and poly (lactic acid) blends. J Appl Polym Sci 112(1):345–352
Vainio MH, Karjalainen T, Seppala J et al (1996) Biodegradable lactone copolymers. 1. Characterization and mechanical behavior of epsilon-caprolactone and lactide copolymers. J Appl Polym Sci 59:1281–1288
Patrício T, Bártolo P et al (2013) Thermal stability of PCL/PLA blends produced by physical blending process. Procedia Eng 59:292–297
Yeh J-T, Wu C-J, Tsou C-H, Chai W-L, Chow J-D, Huang C-Y, Chen K-N, Wu C-S et al (2009) Study on the crystallization, miscibility, morphology, properties of poly (lactic acid)/poly (ε-caprolactone) blends. Polym-plast. Tech. Eng. 48(6):571–578
Broz M, VanderHart DL, Washburn N et al (2003) Structure and mechanical properties of poly (D, L-lactic acid)/poly (ε-caprolactone) blends. Biomaterials 24(23):4181–4190
Semba T, Kitagawa K, Ishiaku US, Hamada H et al (2006) The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J Appl Polym Sci 101(3):1816–1825
Rao RU, Suman KNS, Rao VVSK, Bhanukıran K et al (2011) Study of rheological and mechanical properties of biodegradable polylactide and polycaprolactone blends. Int J Eng Sci Tech 6259–6265
Coleman MM, Serman CJ, Bhagwagar DE, Painter PC et al (1990) A practical guide to polymer miscibility. Polymer 31(7):1187–1203
Meredith JC, Amis EJ et al (2000) LCST phase separation in biodegradable polymer blends: poly(D, L-lactide) and poly(ϵ-caprolactone). Macromol Chem Phys 201(6):733–739
Yang J-M, Chen H-L, You J-W, Hwang JC et al (1997) Miscibility and Crystallization of Poly(L-lactide)/Poly(ethylene glycol) and Poly(L-lactide)/Poly(ε-caprolactone) Blends. Polymer J 29(8):657–662
Navarro-Baena I, Sessini V, Dominici F, Torre L, Kenny JM, Peponi L et al (2016) Design of biodegradable blends based on PLA and PCL: From morphological, thermal and mechanical studies to shape memory behavior. Polym Degrad Stab 132:97–108
Doganci E, Gorur M, Uyanik C, Yilmaz F et al (2014) Synthesis of AB 3-type miktoarm star polymers with steroid core via a combination of “Click” chemistry and ring opening polymerization techniques. J Polym Sci Part A: Polym Chem 52(23):3390–3399
Qin Y, Liu S, Zhang Y, Yuan M, Li H, Yuan M et al (2014) Effect of poly (ɛ-caprolactone-co-L-lactide) on thermal and functional properties of poly (L-lactide). Int J Biol Macromol 70:327–333
Deokar MD, Idage SB, Idage BB, Sivaram S et al (2016) Synthesis and characterization of well-defined random and block copolymers of ε-caprolactone with l-lactide as an additive for toughening polylactide: Influence of the molecular architecture. J Appl Polym Sci 133(14):43267–43279
Li H, Qiao T, Song P, Guo H, Song X, Zhang B, Chen X et al (2015) Star-shaped PCL/PLLA blended fiber membrane via electrospinning. J Biomater Sci, Polym Ed 26(7):420–432
Doganci E, Davarci D et al (2019) Synthesized and mesomorphic properties of cholesterol end-capped poly (ε-caprolactone) polymers. J Polym Res 26(7):165–175
Doganci E, Gorur M, Uyanik C, Yilmaz F et al (2014) Supramolecular inclusion complexes of a star polymer containing cholesterol end-capped poly (ε-caprolactone) arms with β-cyclodextrin J Polym Sci. Part A: Polym Chem 52(23):3406–3420
Blackwell CJ, Haernvall K, Guebitz GM, Groombridge M, Gonzales D, Khosravi E et al (2018) Enzymatic degradation of star poly (ε-caprolactone) with different central units. Polymers 10(11):1266–1281
Choi J, Kim I-K, Kwak S-Y et al (2005) Synthesis and characterization of a series of star-branched poly (ε-caprolactone) s with the variation in arm numbers and lengths. Polymer 46(23):9725–9735
Wang J-L, Dong C-M et al (2006) Physical properties, crystallization kinetics, and spherulitic growth of well-defined poly(ε-caprolactone)s with different arms. Polymer 47(9):3218–3228
Doganci MD, Aynali F, Doganci E, Ozkoc G et al (2019) Mechanical, thermal and morphological properties of poly (lactic acid) by using star-shaped poly (ε-caprolactone) with POSS core. Eur Polym J 121:109316–109329
Dogan SK, Reyes EA, Rastogi S, Ozkoc G et al (2014) Reactive compatibilization of PLA/TPU blends with a diisocyanate. J Appl Polym Sci 131(10):40251–40261
Wang H, Sun X, Seib P et al (2001) Strengthening blends of poly (lactic acid) and starch with methylenediphenyl diisocyanate. J Appl Polym Sci 82(7):1761–1767
Eren O, Gorur M, Keskin B, Yilmaz F et al (2013) Synthesis and characterization of ferrocene end-capped poly (ε-caprolactone) s by a combination of ring-opening polymerization and “click” chemistry techniques. React Funct Polym 73(1):244–253
Şahin ZM, Doğancı E, Yıldız SZ, Tuna M, Yılmaz F, Yerli Y, Görür M et al (2010) Synthesis and characterization of two-armed poly (ɛ-caprolactone) polymers initiated by the Schiff’s base complexes of copper (II) and nickel (II). Synth Met 160(17–18):1973–1980
Aoki D, Uchida S, Takata T et al (2015) Star/Linear Polymer Topology Transformation Facilitated by Mechanical Linking of Polymer Chains. Angew Chem Int Ed 54(23):6770–6774
Kodal M, Sirin H, Ozkoc G et al (2014) Effects of reactive and nonreactive POSS types on the mechanical, thermal, and morphological properties of plasticized poly (lactic acid). Polym Eng Sci 54(2):264–275
Torres N, Robin J, Boutevin B et al (2001) Chemical modification of virgin and recycled poly (ethylene terephthalate) by adding of chain extenders during processing. J Appl Polym Sci 79(10):1816–1824
Kilic NT, Can BN, Kodal M, Ozkoc G et al (2019) Compatibilization of PLA/PBAT blends by using Epoxy-POSS. J Appl Polym Sci 136(12):47217
Zhao H, Zhao G et al (2016) Mechanical and thermal properties of conventional and microcellular injection molded poly (lactic acid)/poly (ε-caprolactone) blends. J Mech Behav Biomed Mater 53:59–67
Monticelli O, Calabrese M, Gardella L, Fina A, Gioffredi E et al (2014) Silsesquioxanes: novel compatibilizing agents for tuning the microstructure and properties of PLA/PCL immiscible blends. Eur Polym J 58:69–78
Matta A, Rao RU, Suman K, Rambabu V et al (2014) Preparation and characterization of biodegradable PLA/PCL polymeric blends. Procedia Mater Sci 6:1266–1270
Migliaresi C, Cohn D, De Lollis A, Fambri L et al (1991) Dynamic mechanical and calorimetric analysis of compression-molded PLLA of different molecular weights: Effect of thermal treatments. J Appl Polym Sci 43(1):83–95
Lim LT, Auras R, Rubino M et al (2008) Processing technologies for poly(lactic acid). Prog Polym Sci 33(8):820–852
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Doganci, M.D. Effects of star-shaped PCL having different numbers of arms on the mechanical, morphological, and thermal properties of PLA/PCL blends. J Polym Res 28, 11 (2021). https://doi.org/10.1007/s10965-020-02380-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02380-2