Abstract
Purpose
To evaluate the factors that affect the incidence of euploid balanced embryos and interchromosomal effect (ICE) in carriers of different structural rearrangements.
Methods
This retrospective study includes 95 couples with reciprocal translocations (RecT) and 36 couples with Robertsonian translocations (RobT) undergoing Preimplantation Genetic Testing for Structural Rearrangements (PGT-SR) between March 2016 and July 2019. Next-generation sequencing (NGS) was the technique used coupled with trophectoderm (TE) biopsy. Only cases with females under 38 years were included. A total of 532 blastocysts were evaluated.
Results
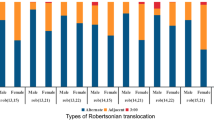
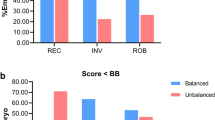
The euploidy rate was similar in RobT when compared with RecT carriers [57/156 (36.5%) vs. 112/376 (29.8%), p = 0.127]. The pure ICE rate was significantly higher in RobT carriers [48/156 (30.8%) vs. 53/376 (14.1%), p < 0.001] than it was in RecT carriers. Female age was the independent factor for the probability of obtaining a euploid embryo in RecT and RobT carriers, and increasing female age decreases the probability of obtaining a euploid embryo. In RecT carriers, no significant differences were observed in euploidy rates, pure ICE, or combined ICE according to the length of the translocated fragment and the chromosome group. However, total ICE was significantly lower when there was a breakpoint in the short chromosome arm together with a breakpoint in the long arm [(44/158 (27.8%) for pq or qp, 51/155 (32.9%) for pp and 30/63 (47.6%) for qq; p = 0.02].
Conclusion
The incidence of euploid/balanced blastocysts was similar in both types of translocations. However, there was a significant increase in pure ICE in RobT compared to RecT carriers. In RecT carriers, the presence of the breakpoints in the long arm of the chromosomes involved in the rearrangement resulted in a higher total ICE.



Similar content being viewed by others
References
Van Dyke DL, Weiss L, Roberson JR, Babu VR. The frequency and mutation rate of balanced autosomal rearrangements in man estimated from prenatal genetic studies for advanced maternal age. Am J Hum Genet. 1983;35(2):301–8.
Vasilevska M, Ivanovska E, Kubelka Sabit K, Sukarova-Angelovska E, Dimeska G. The incidence and type of chromosomal translocations from prenatal diagnosis of 3800 patients in the republic of macedonia. Balkan J Med Genet: BJMG. 2013;16(2):23–8. https://doi.org/10.2478/bjmg-2013-0027.
Scriven PN, Bint SM, Davies AF, Ogilvie CM. Meiotic outcomes of three-way translocations ascertained in cleavage-stage embryos: refinement of reproductive risks and implications for PGD. Eur J Human Genet: EJHG. 2014;22(6):748–53. https://doi.org/10.1038/ejhg.2013.237.
Neri G, Serra A, Campana M, Tedeschi B. Reproductive risks for translocation carriers: cytogenetic study and analysis of pregnancy outcome in 58 families. Am J Med Genet. 1983;16(4):535–61. https://doi.org/10.1002/ajmg.1320160412.
Scriven PN, Flinter FA, Khalaf Y, Lashwood A, Mackie Ogilvie C. Benefits and drawbacks of preimplantation genetic diagnosis (PGD) for reciprocal translocations: lessons from a prospective cohort study. Eur J Human Genet: EJHG. 2013;21(10):1035–41. https://doi.org/10.1038/ejhg.2013.9.
Wilton L, Thornhill A, Traeger-Synodinos J, Sermon KD, Harper JC. The causes of misdiagnosis and adverse outcomes in PGD. Hum Reprod. 2009;24(5):1221–8. https://doi.org/10.1093/humrep/den488.
Fischer J, Colls P, Escudero T, Munne S. Preimplantation genetic diagnosis (PGD) improves pregnancy outcome for translocation carriers with a history of recurrent losses. Fertil Steril. 2010;94(1):283–9. https://doi.org/10.1016/j.fertnstert.2009.02.060.
Keymolen K, Staessen C, Verpoest W, Liebaers I, Bonduelle M. Preimplantation genetic diagnosis in female and male carriers of reciprocal translocations: clinical outcome until delivery of 312 cycles. Eur J Human Genet: EJHG. 2012;20(4):376–80. https://doi.org/10.1038/ejhg.2011.208.
Ko DS, Cho JW, Park SY, Kim JY, Koong MK, Song IO, et al. Clinical outcomes of preimplantation genetic diagnosis (PGD) and analysis of meiotic segregation modes in reciprocal translocation carriers. Am J Med Genet Part A. 2010;152A(6):1428–33. https://doi.org/10.1002/ajmg.a.33368.
Kyu Lim C, Hyun Jun J, Mi Min D, Lee HS, Young Kim J, Koong MK, et al. Efficacy and clinical outcome of preimplantation genetic diagnosis using FISH for couples of reciprocal and Robertsonian translocations: the Korean experience. Prenat Diagn. 2004;24(7):556–61. https://doi.org/10.1002/pd.923.
Munne S, Morrison L, Fung J, Marquez C, Weier U, Bahce M, et al. Spontaneous abortions are reduced after preconception diagnosis of translocations. J Assist Reprod Genet. 1998;15(5):290–6. https://doi.org/10.1023/a:1022544511198.
Munne S, Sandalinas M, Escudero T, Fung J, Gianaroli L, Cohen J. Outcome of preimplantation genetic diagnosis of translocations. Fertil Steril. 2000;73(6):1209–18. https://doi.org/10.1016/s0015-0282(00)00495-7.
Otani T, Roche M, Mizuike M, Colls P, Escudero T, Munné S. Preimplantation genetic diagnosis significantly improves the pregnancy outcome of translocation carriers with a history of recurrent miscarriage and unsuccessful pregnancies. Reprod BioMed Online. 2006;13(6):869–74. https://doi.org/10.1016/S1472-6483(10)61037-1.
Verlinsky Y, Tur-Kaspa I, Cieslak J, Bernal A, Morris R, Taranissi M, et al. Preimplantation testing for chromosomal disorders improves reproductive outcome of poor-prognosis patients. Reprod BioMed Online. 2005;11(2):219–25. https://doi.org/10.1016/s1472-6483(10)60961-3.
Bono S, Biricik A, Spizzichino L, Nuccitelli A, Minasi MG, Greco E, et al. Validation of a semiconductor next-generation sequencing-based protocol for preimplantation genetic diagnosis of reciprocal translocations. Prenat Diagn. 2015;35(10):938–44. https://doi.org/10.1002/pd.4665.
Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, et al. PGD for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26(7):1925–35. https://doi.org/10.1093/humrep/der082.
Tobler KJ, Brezina PR, Benner AT, Du L, Xu X, Kearns WG. Two different microarray technologies for preimplantation genetic diagnosis and screening, due to reciprocal translocation imbalances, demonstrate equivalent euploidy and clinical pregnancy rates. J Assist Reprod Genet. 2014;31(7):843–50. https://doi.org/10.1007/s10815-014-0230-3.
Wang L, Cram DS, Shen J, Wang X, Zhang J, Song Z, et al. Validation of copy number variation sequencing for detecting chromosome imbalances in human preimplantation embryos. Biol Reprod. 2014;91(2):37. https://doi.org/10.1095/biolreprod.114.120576.
Lejeune J. Autosomal disorders. Pediatrics. 1963;32:326–37.
Mateu-Brull E, Rodrigo L, Peinado V, Mercader A, Campos-Galindo I, Bronet F, et al. Interchromosomal effect in carriers of translocations and inversions assessed by preimplantation genetic testing for structural rearrangements (PGT-SR). J Assist Reprod Genet. 2019;36(12):2547–55. https://doi.org/10.1007/s10815-019-01593-9.
Jalbert P, Sele B, Jalbert H. Reciprocal translocations: a way to predict the mode of imbalanced segregation by pachytene-diagram drawing. Hum Genet. 1980;55(2):209–22. https://doi.org/10.1007/BF00291769.
Borm G, Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. The European Orgalutran Study Group. Human Reprod. 2000;15(7):1490–8. https://doi.org/10.1093/humrep/15.7.1490.
Serdarogullari M, Coban O, Boynukalin FK, Bilgin EM, Findikli N, Bahceci M. Successful application of a single warming protocol for embryos cryopreserved by either slow freezing or vitrification techniques. Syst Biol Reprod Med. 2019;65(1):12–9. https://doi.org/10.1080/19396368.2018.1487477.
Zhao H, Tao W, Li M, Liu H, Wu K, Ma S. Comparison of two protocols of blastocyst biopsy submitted to preimplantation genetic testing for aneuploidies: a randomized controlled trial. Arch Gynecol Obstet. 2019;299(5):1487–93. https://doi.org/10.1007/s00404-019-05084-1.
Faraut T, Mermet MA, Demongeot J, Cohen O. Cooperation of selection and meiotic mechanisms in the production of imbalances in reciprocal translocations. Cytogenet Cell Genet. 2000;88(1-2):15–21. https://doi.org/10.1159/000015476.
Anton E, Blanco J, Egozcue J, Vidal F. Sperm FISH studies in seven male carriers of Robertsonian translocation t(13;14)(q10;q10). Human Reprod. 2004;19(6):1345–51. https://doi.org/10.1093/humrep/deh232.
Anton E, Blanco J, Vidal F. Meiotic behavior of three D;G Robertsonian translocations: segregation and interchromosomal effect. J Hum Genet. 2010;55(8):541–5. https://doi.org/10.1038/jhg.2010.67.
Anton E, Vidal F, Blanco J. Role of sperm FISH studies in the genetic reproductive advice of structural reorganization carriers. Hum Reprod. 2007;22(8):2088–92. https://doi.org/10.1093/humrep/dem152.
Anton E, Vidal F, Blanco J. Reciprocal translocations: tracing their meiotic behavior. Genet Med Off J Am Coll Med Genet. 2008;10(10):730–8. https://doi.org/10.1097/GIM.0b013e318187760f.
Anton E, Vidal F, Egozcue J, Blanco J. Preferential alternate segregation in the common t(11;22)(q23;q11) reciprocal translocation: sperm FISH analysis in two brothers. Reprod BioMed Online. 2004;9(6):637–44. https://doi.org/10.1016/s1472-6483(10)61774-9.
Baccetti B, Capitani S, Collodel G, Estenoz M, Gambera L, Piomboni P. Infertile spermatozoa in a human carrier of robertsonian translocation 14;22. Fertil Steril. 2002;78(5):1127–30. https://doi.org/10.1016/s0015-0282(02)03379-4.
Baccetti B, Collodel G, Marzella R, Moretti E, Piomboni P, Scapigliati G, et al. Ultrastructural studies of spermatozoa from infertile males with Robertsonian translocations and 18, X, Y aneuploidies. Hum Reprod. 2005;20(8):2295–300. https://doi.org/10.1093/humrep/dei050.
Blanco J, Egozcue J, Clusellas N, Vidal F. FISH on sperm heads allows the analysis of chromosome segregation and interchromosomal effects in carriers of structural rearrangements: results in a translocation carrier, t(5;8)(q33;q13). Cytogenet Cell Genet. 1998;83(3-4):275–80. https://doi.org/10.1159/000015170.
Blanco J, Egozcue J, Vidal F. Interchromosomal effects for chromosome 21 in carriers of structural chromosome reorganizations determined by fluorescence in situ hybridization on sperm nuclei. Hum Genet. 2000;106(5):500–5. https://doi.org/10.1007/s004390000295.
Chen Y, Huang J, Liu P, Qiao J. Analysis of meiotic segregation patterns and interchromosomal effects in sperm from six males with Robertsonian translocations. J Assist Reprod Genet. 2007;24(9):406–11. https://doi.org/10.1007/s10815-007-9137-6.
Machev N, Gosset P, Warter S, Treger M, Schillinger M, Viville S. Fluorescence in situ hybridization sperm analysis of six translocation carriers provides evidence of an interchromosomal effect. Fertil Steril. 2005;84(2):365–73. https://doi.org/10.1016/j.fertnstert.2005.03.026.
Mercier S, Morel F, Fellman F, Roux C, Bresson JL. Molecular analysis of the chromosomal equipment in spermatozoa of a 46, XY, t(7;8) (q11.21;cen) carrier by using fluorescence in situ hybridization. Hum Genet. 1998;102(4):446–51. https://doi.org/10.1007/s004390050719.
Morel F, Douet-Guilbert N, Roux C, Tripogney C, Le Bris MJ, De Braekeleer M, et al. Meiotic segregation of a t(7;8)(q11.21;cen) translocation in two carrier brothers. Fertil Steril. 2004;81(3):682–5. https://doi.org/10.1016/j.fertnstert.2003.07.034.
Morel F, Roux C, Bresson JL. FISH analysis of the chromosomal status of spermatozoa from three men with 45,XY,der(13;14)(q10;q10) karyotype. Mol Hum Reprod. 2001;7(5):483–8. https://doi.org/10.1093/molehr/7.5.483.
Oliver-Bonet M, Navarro J, Codina-Pascual M, Carrera M, Egozcue J, Benet J. Meiotic segregation analysis in a t(4;8) carrier: comparison of FISH methods on sperm chromosome metaphases and interphase sperm nuclei. Eur J Human Genet: EJHG. 2001;9(6):395–403. https://doi.org/10.1038/sj.ejhg.5200654.
Rousseaux S, Chevret E, Monteil M, Cozzi J, Pelletier R, Delafontaine D, et al. Sperm nuclei analysis of a Robertsonian t(14q21q) carrier, by FISH, using three plasmids and two YAC probes. Hum Genet. 1995;96(6):655–60. https://doi.org/10.1007/BF00210294.
Rousseaux S, Chevret E, Monteil M, Cozzi J, Pelletier R, Devillard F, et al. Meiotic segregation in males heterozygote for reciprocal translocations: analysis of sperm nuclei by two and three colour fluorescence in situ hybridization. Cytogenet Cell Genet. 1995;71(3):240–6. https://doi.org/10.1159/000134118.
Van Hummelen P, Manchester D, Lowe X, Wyrobek AJ. Meiotic segregation, recombination, and gamete aneuploidy assessed in a t(1;10)(p22.1;q22.3) reciprocal translocation carrier by three- and four-probe multicolor FISH in sperm. Am J Hum Genet. 1997;61(3):651–9. https://doi.org/10.1086/515516.
Vozdova M, Oracova E, Musilova P, Kasikova K, Prinosilova P, Gaillyova R, et al. Sperm and embryo analysis of similar t(7;10) translocations transmitted in two families. Fertil Steril. 2011;96(1):e66–70. https://doi.org/10.1016/j.fertnstert.2011.04.042.
Wiland E, Midro AT, Panasiuk B, Kurpisz M. The analysis of meiotic segregation patterns and aneuploidy in the spermatozoa of father and son with translocation t(4;5)(p15.1;p12) and the prediction of the individual probability rate for unbalanced progeny at birth. J Androl. 2007;28(2):262–72. https://doi.org/10.2164/jandrol.106.000919.
Juchniuk de Vozzi MS, Santos SA, Pereira CS, Cuzzi JF, Laureano LA, Franco JG Jr, et al. Meiotic segregation and interchromosomal effect in the sperm of a double translocation carrier: a case report. Mol Cytogenet. 2009;2:24. https://doi.org/10.1186/1755-8166-2-24.
Pellestor F, Imbert I, Andreo B, Lefort G. Study of the occurrence of interchromosomal effect in spermatozoa of chromosomal rearrangement carriers by fluorescence in-situ hybridization and primed in-situ labelling techniques. Human Reproduction. 2001;16(6):1155–64. https://doi.org/10.1093/humrep/16.6.1155.
Chelli MH, Ferfouri F, Boitrelle F, Albert M, Molina-Gomes D, Selva J, et al. High-magnification sperm selection does not decrease the aneuploidy rate in patients who are heterozygous for reciprocal translocations. J Assist Reprod Genet. 2013;30(4):525–30. https://doi.org/10.1007/s10815-013-9959-3.
Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E. Is there an interchromosomal effect in reciprocal translocation carriers? Sperm FISH studies. Hum Genet. 2000;106(5):517–24. https://doi.org/10.1007/s004390000275.
Godo A, Blanco J, Vidal F, Sandalinas M, Garcia-Guixe E, Anton E. Altered segregation pattern and numerical chromosome abnormalities interrelate in spermatozoa from Robertsonian translocation carriers. Reprod BioMed Online. 2015;31(1):79–88. https://doi.org/10.1016/j.rbmo.2015.04.003.
Honda H, Miharu N, Ohashi Y, Honda N, Hara T, Ohama K. Analysis of segregation and aneuploidy in two reciprocal translocation carriers, t(3;9)(q26.2;q32) and t(3;9)(p25;q32), by triple-color fluorescence in situ hybridization. Hum Genet. 1999;105(5):428–36. https://doi.org/10.1007/s004390051126.
Pujol A, Durban M, Benet J, Boiso I, Calafell JM, Egozcue J, et al. Multiple aneuploidies in the oocytes of balanced translocation carriers: a preimplantation genetic diagnosis study using first polar body. Reproduction. 2003;126(6):701–11. https://doi.org/10.1530/rep.0.1260701.
Tulay P, Gultomruk M, Findikli N, Bahceci M. Number of embryos biopsied as a predictive indicator for the outcome of preimplantation genetic diagnosis by fluorescence in situ hybridisation in translocation cases. Zygote. 2016;24(1):107–14. https://doi.org/10.1017/S0967199414000793.
Munne S, Escudero T, Fischer J, Chen S, Hill J, Stelling JR, et al. Negligible interchromosomal effect in embryos of Robertsonian translocation carriers. Reprod BioMed Online. 2005;10(3):363–9. https://doi.org/10.1016/s1472-6483(10)61797-x.
Alfarawati S, Fragouli E, Colls P, Wells D. Embryos of robertsonian translocation carriers exhibit a mitotic interchromosomal effect that enhances genetic instability during early development. PLoS Genet. 2012;8(10):e1003025. https://doi.org/10.1371/journal.pgen.1003025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report related with this manuscript.
Ethical approval
This study was approved by the Institutional Review Board with a reference number of 55.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Boynukalin, F.K., Gultomruk, M., Turgut, N.E. et al. The impact of patient, embryo, and translocation characteristics on the ploidy status of young couples undergoing preimplantation genetic testing for structural rearrangements (PGT-SR) by next generation sequencing (NGS). J Assist Reprod Genet 38, 387–396 (2021). https://doi.org/10.1007/s10815-020-02054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-02054-4