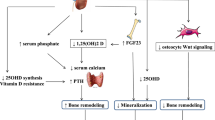
Abstract
Abnormal bone metabolism is an integral part of the chronic kidney disease-mineral bone disorder (CKD-MBD). For several reasons, the difficult bone compartment was neglected for some time, but there has been renewed interest as a result of the conception of bone as a new endocrine organ, the increasing recognition of the cross-talk between bone and vessels, and, especially, the very high risk of osteoporotic fractures (and associated mortality) demonstrated in patients with CKD. Therefore, it has been acknowledged in different guidelines that action is needed in respect of fracture risk assessment and the diagnosis and treatment of osteoporosis in the context of CKD and CKD-MBD, even beyond renal osteodystrophy. These updated guidelines clearly underline the need to improve a non-invasive approach to these bone disorders in order to guide treatment decisions aimed at not only controlling CKD-MBD but also decreasing the risk of fracture. In this report, we review the current role of the most often clinically used or promising biochemical circulating biomarkers such as parathyroid hormone, alkaline phosphatases, and other biochemical markers of bone activity as alternatives to some aspects of bone histomorphometry. We also mention the potential role of classic and new imaging techniques for CKD patients. Information on many aspects is still scarce and heterogeneous, but many of us consider that it is indeed time for action, recognizing our definitely limited ability to base certain treatment decisions only on our current non-comprehensive knowledge.
Similar content being viewed by others
References
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB (2017) Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int 92(1):26–36
Cozzolino M, Ureña-Torres P, Vervloet MG, Brandenburg V, Bover J, Goldsmith D, Larsson TE, Massy ZA, Mazzaferro S, CKD-MBD Working Group of ERA-EDTA (2014) Is chronic kidney disease-mineral bone disorder (CKD-MBD) really a syndrome? Nephrol Dial Transplant. 29(10):1815–1820
Torres PU, Bover J, Mazzaferro S, de Vernejoul MC, Cohen-Solal M (2014) When, how, and why a bone biopsy should be performed in patients with chronic kidney disease. Semin Nephrol 34(6):612–625
Llach F, Bover J (2000) Renal osteodystrophies. In: Brenner BM (ed) The kidney, 6th edn. W.B. Saunders Company, Philadelphia, pp 2103–2186
Liu SH, Chu HI (1942) Treatment of renal osteodystrophy with dihydrotachysterol (a.t.10) and iron. Science (Washington) 95(2467):388–389
Lucas RC (1883) On a form of late rickets associated with albuminuria, rickets of adolescence. Lancet 1:993–994
Fletcher HM (1910) Case of infantilism with polyuria and chronic renal disease. Proc Roy Soc Med Sect Stud Dis Child 11(4):95
Langmead FS, Orr JW (1933) Renal rickets associated with parathyroid hyperplasia. Arch Dis Child 8:265–278
Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G, Kidney Disease: Improving Global Outcomes (KDIGO) (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69(11):1945–1953
Vervloet MG, Massy ZA, Brandenburg VM, Mazzaferro S, Cozzolino M, Ureña-Torres P, Bover J, Goldsmith D, CKD-MBD Working Group of ERA-EDTA (2014) Bone: a new endocrine organ at the heart of chronic kidney disease and mineral and bone disorders. Lancet Diabetes Endocrinol 2(5):427–436
Covic A, Vervloet M, Massy ZA, Ureña-Torres P, Goldsmith D, Brandenburg V, Mazzaferro S, Evenepoel P, Bover J, Apetri M, Cozzolino M (2018) Bone and mineral disorders in chronic kidney disease: implications for cardiovascular health and ageing in the general population. Lancet Diabetes Endocrinol 6(4):319–331
Mazzaferro S, De Martini N, Rotondi S, Tartaglione L, Ureña-Torres P, Bover J, Pasquali M, ERA-EDTA Working Group on CKD-MBD (2020) Bone, inflammation and chronic kidney disease. Clin Chim Acta. 506:236–240
Mazzaferro S, Cianciolo G, De Pascalis A, Guglielmo C, Urena Torres PA, Bover J, Tartaglione L, Pasquali M, La Manna G (2018) Bone, inflammation and the bone marrow niche in chronic kidney disease: what do we know? Nephrol Dial Transplant 33(12):2092–2100
Moe SM, Nickolas TL (2016) Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol 11(11):1929–1931
Goldenstein PT, Jamal SA, Moysés RMA (2015) Fractures in chronic kidney disease: pursuing the best screening and management. Curr Opin Nephrol Hypertens 24(4):317–323
Bover J, Ureña-Torres P, Torregrosa JV, Rodríguez-García M, Castro-Alonso C, Górriz JL, Laiz Alonso AM, Cigarrán S, Benito S, López-Báez V, Lloret Cora MJ, daSilva I, Cannata-Andía J (2018) Osteoporosis, bone mineral density and CKD-MBD complex (I): diagnostic considerations. Nefrologia 38(5):476–490
Bover J, Ureña-Torres P, Laiz Alonso AM, Torregrosa JV, Rodríguez-García M, Castro-Alonso C, Górriz JL, Benito S, López-Báez V, Lloret Cora MJ, Cigarrán S, DaSilva I, Sánchez-Bayá M, Mateu Escudero S, Guirado L, Cannata-Andía J (2019) Osteoporosis, bone mineral density and CKD-MBD (II): therapeutic implications. Nefrologia 39(3):227–242
Torregrosa V, Bover J, Rodriguez M et al (2020) Spanish Society of Nephrology recommendations for controlling mineral and bone disorders in chronic kidney disease patients. Nefrologia 31:3–32
Bover J, Bailone L, López-Báez V, Benito S, Ciceri P, Galassi A, Cozzolino M (2017) Osteoporosis, bone mineral density and CKD-MBD: treatment considerations. J Nephrol 30(5):677–687
Evenepoel P, Cunningham J, Ferrari S, Haarhaus M, Javaid MK, Lafage-Proust MH, Prieto-Alhambra D, Torres PU, Cannata-Andia J (2020) European Renal Osteodystrophy (EUROD) workgroup, an initiative of the CKD-MBD working group of the ERA-EDTA, and the committee of Scientific Advisors and National Societies of the IOF. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfaa192
Nickolas TL, McMahon DJ, Shane E (2006) Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17:3223–3232
Ishani A, Blackwell T, Jamal SA et al (2008) The effect of raloxifene treatment in postmenopausal women with CKD. J Am Soc Nephrol 19:1430–1438
Jamal SA, Bauer DC, Ensrud KE et al (2007) Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res 22:503–508
Miller PD, Roux C, Boonen S et al (2005) Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res 20:2105–2115
Miller PD, Schwartz EN, Chen P et al (2007) Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int 18:59–68
Jamal SA, Ljunggren O, Stehman-Breen C et al (2011) Effects of denosumab on fracture and bone mineral density by level of kidney function. J Bone Miner Res 26:1829–1835
Vervloet MG, Brandenburg VM, CKD-MBD working group of ERA-EDTA (2017) Circulating markers of bone turnover. J Nephrol 30(5):663–670
Bover J, Ureña P, Aguilar A, Mazzaferro S, Benito S, López-Báez V, Ramos A, daSilva I, Cozzolino M (2018) Alkaline phosphatases in the complex chronic kidney disease-mineral and bone disorders. Calcif Tissue Int 103(2):111–124
Ureña-Torres P, Hruby M, Ferreira A, Ang KS, de Vernejoul MC (1996) Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol 7(3):506–512
Ureña-Torres P, de Vernejoul C (1999) Circulating biochemical markers of bone remodelling in uremic patients. Kidney Int 55(6):2141–2156
National Kidney Foundation (2003) K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42(4 Suppl 3):S1-201
Nizet A, Cavalier E, Stenvinkel P, Haarhaus M, Magnusson P (2020) Bone alkaline phosphatase: an important biomarker in chronic kidney disease—mineral and bone disorder. Clin Chim Acta 501:198–206
Couttenye M, D’Haese PC, Van Hoof VO, Lemoniatou E, Goodman W, Verpooten GA, De Broe ME (1996) Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant 11(6):1065–1072
Ureña-Torres PA, Bover J, Cohen-Solal M (2020) Relation between PTH and biochemical markers of MBD. In: Covic A, Goldsmith G, Ureña-Torres PA (eds) Parathyroid glands in chronic kidney disease. Berlin, Springer, pp 103–116
Lau WL, Kalantar-Zadeh K (2014) Towards the revival of alkaline phosphatase for the management of bone disease, mortality and hip fractures. Nephrol Dial Transplant 29(8):1450–1452
Coen G, Ballanti P, Bonucci E, Calabria S, Centorrino M, Fassino V, Manni M, Mantella D, Mazzaferro S, Napoletano I, Sardella D, Taggi F (1998) Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant 13(9):2294–2302
Behets GJ, Spasovski G, Sterling LR, Goodman WG, Spiegel DM, De Broe ME, D’Haese PC (2015) Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 87(4):846–856
Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, D’Haese PC, Drüeke TB, Du H, Manley T, Rojas E, Moe SM (2016) Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis 67(4):559–566
Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, Malluche HH (2001) Improved assessment of bone turnover by the PTH-(1–84)/large C-PTH fragments ratio in ESRD patients. Kidney Int 60(4):1460–1468
Ardawi MSM, Rouzi AA, Qari MH (2012) Physical activity in relation to serum sclerostin, insulin-like growth factor-1, and bone turnover markers in healthy premenopausal women: a cross-sectional and a longitudinal study. J Clin Endocrinol Metab 97(10):3691–3699
Sardiwal S, Gardham C, Coleman AE, Stevens PE, Delaney MP, Lamb EJ (2012) Bone-specific alkaline phosphatase concentrations are less variable than those of parathyroid hormone in stable hemodialysis patients. Kidney Int 82(1):100–105
Mazzaferro S, Tartaglione L, Rotondi S, Bover J, Goldsmith D, Pasquali M (2014) News on biomarkers in CKD-MBD. Semin Nephrol 34(6):598–611
Haarhaus M, Monier-Faugere MC, Magnusson P, Malluche HH (2015) Bone alkaline phosphatase isoforms in hemodialysis patients with low versus non-low bone turnover: a diagnostic test study. Am J Kidney Dis 66(1):99–105
Kim DW, Hwang SY, Nam YJ, Kim D, Shin SJ, Yoon HE (2020) The combined prognostic significance of alkaline phosphatase and vascular calcification in patients with end-stage kidney disease. Nutr Metab Cardiovasc Dis 30(9):1476–1483
Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P (2017) Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol 13(7):429–442
Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, Massart C, Monge M, Myara J, Parent X, Plouvier E, Houillier P (2006) Inter-method variability in PTH. Kidney Int 70:345–350
Floege J, Kim J, Ireland E, Chazot Ch, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC, ARO Investigators (2011) Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26(6):1948–1955
Naves-Díaz M, Passlick-Deetjen J, Guinsburg A, Marelli C, Fernández-Martín JL, Rodríguez-Puyol D, Cannata-Andía JB (2011) Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant 26(6):1938–1947
Herberth J, Branscum AJ, Mawad H, Cantor T, Monier-Faugere MC, Malluche HH (2010) Intact PTH combined with the PTH ratio for diagnosis of bone turnover in dialysis patients: a diagnostic test study. Am J Kidney Dis 55(5):897–906
Sawaya BP, Butros R, Naqv S, Geng Z, Mawad H, Friedler R, Fanti P, Monier-Faugere MC, Malluche HH (2003) Differences in bone turnover and intact PTH levels between African American and Caucasian patients with end-stage renal disease. Kidney Int 64(2):737–742
Moore C, Yee J, Malluche H, Rao DS, Monier-Faugere MC, Adams E, Daramola-Ogunwuyi O, Fehmi H, Bhat S, Osman-Malik Y (2009) Relationship between bone histology and markers of bone and mineral metabolism in African-American hemodialysis patients. Clin J Am Soc Nephrol 4(9):1484–1493
Hocher B, Armbruster FP, Stoeva S, Reichetzeder C, Grön HJ, Lieker I, Khadzhynov D, Slowinski T, Roth HJ (2012) Measuring parathyroid hormone (PTH) in patients with oxidative stress–do we need a fourth generation parathyroid hormone assay? PLoS ONE 7(7):e40242
Sprague SM, Moe SM (2013) The case for routine parathyroid hormone monitoring. Clin J Am Soc Nephrol 8(2):313–318
Garrett G, Sardiwal S, Lamb EJ, Goldsmith DJA (2013) PTH–a particularly tricky hormone: why measure it at all in kidney patients? Clin J Am Soc Nephrol 8(2):299–312
Bover J, Ureña P, Brandenburg V, Goldsmith D, Ruiz C, DaSilva I, Bosch RJ (2014) Adynamic bone disease: from bone to vessels in chronic kidney disease. Semin Nephrol 34(6):626–640
Díaz-Tocados JM, Rodríguez-Ortiz ME, Almadén Y, Pineda C, Martínez-Moreno JM, Herencia C, Vergara N, Pendón-Ruiz de Mier MV, Santamaría R, Rodelo-Haad C, Casado-Díaz A, Lorenzo V, Carvalho C, Frazão JM, Felsenfeld AF, Richards WG, Aguilera-Tejero E, Mariano Rodríguez M, López I, Muñoz-Castañeda JR (2019) Calcimimetics maintain bone turnover in uremic rats despite the concomitant decrease in parathyroid hormone concentration. Kidney Int 95(5):1064–1078
Mazzaferro S, Pasquali M (2019) Direct bone effects of calcimimetics in chronic kidney disease? Kidney Int 95(5):1012–1026
Li JL, Yu M, Pal S, Tyagi AM, Dar H, Adams J, Weitzmann MN, Jones RM, Pacifici R (2020) Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest 130(4):1767–1781
Moorthi RN, Moe SM (2013) Recent advances in the noninvasive diagnosis of renal osteodystrophy. Kidney Int 84(5):886–894
Evenepoel P, Cavalier E, D’Haese PC (2017) Biomarkers predicting bone turnover in the setting of CKD. Curr Osteoporos Rep 15(3):178–186
Evenepoel P, Claes K, Meijers B, Laurent MR, Bammens B, Naesens M, Sprangers B, Pottel H, Cavalier E, Kuypers D (2019) Bone mineral density, bone turnover markers, and incident fractures in de novo kidney transplant recipients. Kidney Int 95(6):1461–1470
Eastell R, Szulc P (2017) Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol 5(11):908–923
Ureña-Torres PA, Vervloet M, Mazzaferro S, Oury F, Brandenburg V, Bover J, Cavalier E, Cohen-Solal M, Covic A, Drüeke TB, Hindié E, Evenepoel P, Frazão J, Goldsmith D, Kazama JJ, Cozzolino M, Massy ZA, ERA-EDTA CKD-MBD Working Group (2018) Novel insights into parathyroid hormone: report of The Parathyroid Day in Chronic Kidney Disease. Clin Kidney J 12(2):269–280
Reiss AB, Miyawaki N, Moon J, Kasselman LJ, Voloshyna I, D’Avino R, de Leon J (2018) CKD, arterial calcification, atherosclerosis and bone health: interrelationships and controversies. Atherosclerosis 278:49–59
Alvarez L, Torregrosa JV, Peris P, Monegal A, Bedini JL, Martinez De Osaba MJ, Filella X, Martin G, Ricos C, Oppenheimer F, Ballesta AM (2004) Effect of hemodialysis and renal failure on serum biochemical markers of bone turnover. J Bone Miner Metab 22(3):254–259
Ueda M, Inaba M, Okuno S, Nagasue K, Kitatani K, Ishimura E, Shimizu M, Miki T, Kim M, Nishizawa Y (2002) Clinical usefulness of the serum N-terminal propeptide of type I collagen as a marker of bone formation in hemodialysis patients. Am J Kidney Dis 40(4):802–809
Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, Chauncey R, Nikkel L, Yin MT, Liu XS, Boutroy S, Staron RB, Leonard MB, McMahon DJ, Dworakowski E, Shane E (2011) Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol 22(8):1560–1572
Nagata Y, Inaba M, Imanishi Y, Okazaki H, Yamada S, Mori K, Shoji S, Koyama H, Okuno S (2015) Increased undercarboxylated osteocalcin/intact osteocalcin ratio in patients undergoing hemodialysis. Osteoporos Int. 26(3):1053–1061
Marchelek-Mysliwiec M, Wisniewska M, Nowosiad-Magda M, Safranow K, Kwiatkowska E, Banach B, Dołegowska B, Dołegowska K, Stepniewska J, Domanski L, Pawlik A, Ciechanowski K (2018) Association between plasma concentration of klotho protein, osteocalcin, leptin, adiponectin, and bone mineral density in patients with chronic kidney disease. Horm Metab Res 50(11):816–821
Henriksen K, Tanko LB, Qvist P, Delmas PD, Christiansen C, Karsdal MA (2007) Assessment of osteoclast number and function: application in the development of new and improved treatment modalities for bone diseases. Osteoporos Int 18(5):681–685
Shidara K, Inaba M, Okuno S, Yamada S, Kumeda Y, Imanishi Y, Yamakawa T, Ishimura E, Nishizawa Y (2008) Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif Tissue Int 82(4):278–287
Chu P, Chao TY, Lin YF, Janckila AJ, Yam LT (2003) Correlation between histomorphometric parameters of bone resorption and serum type 5b tartrate-resistant acid phosphatase in uremic patients on maintenance hemodialysis. Am J Kidney Dis 41(5):1052–1059
Fahrleitner-Pammer A, Herberth J, Browning SR, Obermayer-Pietsch B, Wirnsberger G, Holzer H, Dobnig H, Malluche HH (2008) Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res 23(11):1850–1858
Graciolli FG, Neves KR, Barreto F, Barreto DV, Dos Reis LM, Canziani ME, Sabbagh Y, Carvalho AB, Jorgetti V, Elias RM, Schiavi S, Moysés RMA (2017) The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int 91(6):1436–1446
Murali SK, Andrukhova O, Clinkenbeard EL, White KE, Erben RG (2016) Excessive osteocytic Fgf23 secretion contributes to pyrophosphate accumulation and mineralization defect in Hyp mice. PLoS Biol 14(4):e1002427
Vervloet M (2019) Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol 15(2):109–120
Johnson ML, Kamel MA (2007) The Wnt signaling pathway and bone metabolism. Curr Opin Rheumatol 19(4):376–382
Amrein K, Dobnig H, Wagner D, Piswanger-Sölkner C, Pieber TR, Pilz S, Tomaschitz A, Dimai HP, Fahrleitner-Pammer A (2014) Sclerostin in institutionalized elderly women: associations with quantitative bone ultrasound, bone turnover, fractures, and mortality. J Am Geriatr Soc 62(6):1023–1029
Cejka D, Herberth J, Branscum AJ, Fardo DW, Monier-Faugere MC, Diarra D, Haas M, Malluche HH (2011) Sclerostin and Dickkopf-1 in renal osteodystrophy. Clin J Am Soc Nephrol 6(4):877–882
Araújo MJCLN, Bacelar Marques ID, Graciolli FG, Fukuhara L, Machado Dos Reis L, Custódio M, Jorgetti V, Elias RM, David-Neto E, Moysés RMA (2019) Comparison of serum levels with bone content and gene expression indicate a contradictory effect of kidney transplantation on sclerostin. Kidney Int 96(5):1100–1104
Pimentel A, Ureña-Torres P, Zillikens MC, Bover J, Cohen-Solal M (2017) Fractures in patients with CKD-diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 92(6):1343–1355
Kuo TH, Lin WH, Chao JY, Wu AB, Tseng C-C, Chang YT, Liou HH, Wang MC (2019) Serum sclerostin levels are positively related to bone mineral density in peritoneal dialysis patients: a cross-sectional study. BMC Nephrol 20(1):266
Cejka D, Jäger-Lansky A, Kieweg H, Weber M, Bieglmayer C, Haider DG, Diarra D, Patsch JM, Kainberger F, Bohle B, Haas M (2012) Sclerostin serum levels correlate positively with bone mineral density and microarchitecture in haemodialysis patients. Nephrol Dial Transplant 27(1):226–230
Thambiah S, Roplekar R, Manghat P, Fogelman I, Fraser WD, Goldsmith D, Hampson G (2012) Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif Tissue Int 90(6):473–480
Ishimura E, Okuno S, Ichii M, Norimine K, Yamakawa T, Shoji S, Nishizawa Y, Inaba M (2014) Relationship between serum sclerostin, bone metabolism markers, and bone mineral density in maintenance hemodialysis patients. J Clin Endocrinol Metab 99(11):4315–4320
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CAF, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N Engl J Med 375(16):1532–1543
Brandenburg VM, Verhulst A, Babler A, D’Haese PC, Evenepoel P, Kaesler N (2019) Sclerostin in chronic kidney disease-mineral bone disorder think first before you block it! Nephrol Dial Transplant 34(3):408–414
Carrillo-López N, Panizo S, Alonso-Montes C, Román-García P, Rodríguez I, Martínez-Salgado C, Dusso AS, Naves M, Cannata-Andía JB (2016) Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int 90(1):77–89
Sabbagh Y, Graciolli FG, O’Brien S, Tang W, dos Reis LM, Ryan S, Phillips L, Boulanger J, Song W, Bracken C, Liu S, Ledbetter S, Dechow P, Canziani ME, Carvalho AB, Jorgetti V, Moyses RM, Schiavi SC (2012) Repression of osteocyte Wnt/beta-catenin signaling is an early event in the progression of renal osteodystrophy. J Bone Miner Res 27(8):1757–1772
Evenepoel P, Bover J, Ureña-Torres P (2016) Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int 90(6):1184–1190
Staines KA, MacRae VE, Farquharson C (2012) The importance of the SIBLING family of proteins on skeletal mineralisation and bone remodelling. J Endocrinol 214(3):241–255
Rowe PSN (2012) Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit Rev Eukaryot Gene Expr 22(1):61–86
Kong L, Wu H, Zhou W, Luo M, Tan Y, Miao L, Cai L (2015) Sirtuin 1: a target for kidney diseases. Mol Med 21(1):87–97
Zainabadi K, Liu CJ, Guarente L (2017) SIRT1 is a positive regulator of the master osteoblast transcription factor, RUNX2. PLoS ONE 5:e0178520
Feng Q, Zheng S, Zheng J (2018) The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci Rep 38(3):BSR20180453
Iimori S, Mori Y, Akita W, Kuyama T, Takada S, Asai T, Kuwahara M, Sasaki S, Tsukamoto Y (2012) Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients–a single-center cohort study. Nephrol Dial Transplant 27(1):345–351
Naylor KL, Garg AX, Zou G, Langsetmo L, Leslie WD, Fraser LA, Adachi JD, Suzanne Morin S, Goltzman D, Lentle B, Jackson SA, Josse RG, Jamal SA (2015) Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol 10(4):646–653
West SL, Lok CE, Langsetmo L, Cheung AM, Szabo E, Pearce D, Fusaro M, Wald R, Weinstein J, Jamal SA (2015) Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res 30(5):913–919
Yenchek RH, Iuuux JH, Shlipak MG, Bauer DC, Rianon NJ, Kritchevsky SB, Harris TB, Newman AB, Cauley JA, Fried LF (2012) Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol 7(7):1130–1136
Prasad B, Ferguson T, Tangri N, Ng CY, Nickolas TL (2019) Association of bone mineral density with fractures across the spectrum of chronic kidney disease: the regina CKD-MBD study. Can J Kidney Health Dis 6:2054358119870539
Hind K, Oldroyd B, Truscott JG (2010) In vivo precision of the GE Lunar iDXA densitometer for the measurement of total-body, lumbar spine, and femoral bone mineral density in adults. J Clin Densitom 13(4):413–417
Dusceac R, Niculescu DA, Dobre R, Dragne MD, Tacu C, Peride I, David C, Checherita I, Poiana C (2018) Chronic hemodialysis is associated with lower trabecular bone score, independent of bone mineral density: a case-control study. Arch Osteoporos 13(1):125
Naylor KL, Prior J, Garg AX, Berger C, Langsetmo L, Adachi JD, Goltzman D, Kovacs CS, Josse RG, Leslie WD (2016) Trabecular bone score and incident fragility fracture risk in adults with reduced kidney function. Clin J Am Soc Nephrol 11(11):2032–2040
Pocock N (2017) Use of dual energy X-ray absorptiometry, the trabecular bone score and quantitative computed tomography in the evaluation of chronic kidney disease-mineral and bone disorders. Nephrology (Carlton) 22(Suppl 2):19–21
Rampersad C, Whitlock RH, Leslie WD, Rigatto C, Komenda P, Bohm C, Hans D, Tangri N (2020) Trabecular bone score in patients with chronic kidney disease. Osteoporos Int 31(10):1905–1912
Ramalho J, Marques IDB, Hans D, Dempster D, Zhou H, Patel P, Pereira RMR, Jorgetti V, Moyses RMA, Nickolas TL (2018) The trabecular bone score: relationships with trabecular and cortical microarchitecture measured by HR-pQCT and histomorphometry in patients with chronic kidney disease. Bone 116:215–220
Fusaro M, D’Angelo A, Gallieni M (2008) Vertebral fractures in patients on dialysis: a clinically relevant problem with insufficient investigation. NDT Plus 1(6):464–465
Fusaro M, Aghi A, Mereu MC, Giusti A (2017) Fragility fracture in the Chronic Kidney Disease (CKD). G Ital Nefrol 34:2017-vol6
Gracia-Marco L, García-Fontana B, Ubago-Guisado E, Vlachopoulos D, García-Martín A, Muñoz-Torres M (2020) Analysis of bone impairment by 3D DXA hip measures in patients with primary hyperparathyroidism: a pilot study. J Clin Endocrinol Metab 105(1):dgz060
Ghesani N, Jung J, Patel S, Ramchand T (2013) Superscan caused by renal osteodystrophy: Observed on 18F FDG PET/CT scan. Indian J Nucl Med 28(4):251–252
Torres A, Lorenzo V, Gonzalez-Posada JM (1986) Comparison of histomorphometry and computerized tomography of the spine in quantitating trabecular bone in renal osteodystrophy. Nephron 44(4):282–287
Chen Z, Qureshi AR, Ripsweden J, Wennberg L, Heimburger O, Lindholm B, Barany P, Haarhaus M, Brismar TB, Stenvinkel P (2016) Vertebral bone density associates with coronary artery calcification and is an independent predictor of poor outcome in end-stage renal disease patients. Bone 92:50–57
Filgueira A, Carvalho AB, Tomiyama C, Higa A, Rochitte CE, Santos RD, Canziani ME (2011) Is coronary artery calcification associated with vertebral bone density in nondialyzed chronic kidney disease patients? Clin J Am Soc Nephrol 6(6):1456–1462
Jamal SA, Gilbert J, Gordon C, Bauer DC (2006) Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res 21(4):543–548
Jamal S, Cheung AM, West S, Lok C (2012) Bone mineral density by DXA and HR pQCT can discriminate fracture status in men and women with stages 3 to 5 chronic kidney disease. Osteoporos Int 23(12):2805–2813
Bacchetta J, Boutroy S, Vilayphiou N, Juillard L, Guebre-Egziabher F, Rognant N, Sornay-Rendu E, Szulc P, Laville M, Delmas PD, Fouque D, Chapurlat R (2010) Early impairment of trabecular microarchitecture assessed with HR-pQCT in patients with stage II-IV chronic kidney disease. J Bone Miner Res 25(4):849–857
Cejka D, Patsch JM, Weber M, Diarra D, Riegersperger M, Kikic Z, Krestan C, Schueller-Weidekamm C, Kainberger F, Haas M (2011) Bone microarchitecture in hemodialysis patients assessed by HR-pQCT. Clin J Am Soc Nephrol 6(9):2264–2271
Paranhos-Neto FP, Lima GAC, Silva LC, Madeira M, Neto LV, Mendonça LMC, Lima ICB, Delgado AG, Leite M Jr, Gomes CP, Farias MLF (2018) HR-pQCT detects alterations in bone microstructure in men with CKD stages 3 and 4, which are influenced by hormonal changes and body composition. Clin Nephrol 89(1):10–17
Marques ID, Araújo MJ, Graciolli FG, Reis LM, Pereira RM, Custódio MR, Jorgetti V, Elias RM, David-Neto E, Moysés RM (2017) Biopsy vs. peripheral computed tomography to assess bone disease in CKD patients on dialysis: differences and similarities. Osteoporos Int 28(5):1675–1683
Salam S, Gallagher O, Gossiel F, Paggiosi M, Khwaja A, Eastell R (2018) Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol 29(5):1557–1565
Nickolas TL (2018) The quest for better biomarkers of bone turnover in CKD. J Am Soc Nephrol 29:1353–1355
Crandall CJ, Vasan S, LaCroix A, LeBoff MS, Cauley JA, Robbins JA, Jackson RD, Bauer DC (2018) Bone turnover markers are not associated with hip fracture risk: a case-control study in the women’s health initiative. J Bone Miner Res 33(7):1199–1208
Link TM, Saborowski O, Kisters K, Kempkes M, Kosch M, Newitt D, Lu Y, Waldt S, Majumdar S (2002) Changes in calcaneal trabecular bone structure assessed with high-resolution MR imaging in patients with kidney transplantation. Osteoporos Int 13(2):119–129
Wehrli FW, Leonard MB, Saha PK, Gomberg BR (2004) Quantitative high-resolution magnetic resonance imaging reveals structural implications of renal osteodystrophy on trabecular and cortical bone. J Magn Reson Imaging 20(1):83–89
Bover J, Jara A, Trinidad P, Rodriguez M, Martin-Malo A, Felsenfeld AJ (1994) The calcemic response to PTH in the rat: effect of elevated PTH levels and uremia. Kidney Int 46(2):310–317
Bover J, Jara A, Trinidad P, Rodriguez M, Felsenfeld AJ (1999) Dynamics of skeletal resistance to parathyroid hormone in the rat: effect of renal failure and dietary phosphorus. Bone 25(3):279–285
Holloway-Kew KL, Rufus-Membere P, Anderson KB, Betson A, Gaston J, Kotowicz MA, Diez-Perez A, Hyde NK, Pasco JA (2020) Bone material strength index is associated with prior fracture in men with and without moderate chronic kidney disease. Bone 133:115241
Pérez-Sáez MJ, Prieto-Alhambra D, Díez-Pérez A, Pascual J (2018) Advances in the evaluation of bone health in kidney transplant patients. Nefrologia 38(1):27–33
Salam SN, Eastell R, Khwaja A (2014) Fragility fractures and osteoporosis in CKD: pathophysiology and diagnostic methods. Am J Kidney Dis 63(6):1049–1059
Khairallah P, Nickolas TL (2018) Management of osteoporosis in CKD. Clin J Am Soc Nephrol 13(6):962–969
Bover J, Ureña-Torres P, Mateu S, DaSilva I, Gràcia S, Sánchez-Baya M, Arana C, Fayos L, Guirado L, Cozzolino M (2020) Evidence in chronic kidney disease-mineral and bone disorder guidelines: is it time to treat or time to wait? Clin Kidney J 13(4):513–521
Wilson LM, Rebholz CM, Jirru E, Liu MC, Zhang A, Gayleard J, Chu Y, Robinson KA (2017) Benefits and harms of osteoporosis medications in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 166(9):649–658
Hara T, Hijikata Y, Matsubara Y, Watanabe N (2019) Pharmacological interventions for osteoporosis in people with chronic kidney disease stages 3–5D. Cochrane Datab Syst Rev. https://doi.org/10.1002/14651858.CD013424
Acknowledgements
We thank Mr Ricard Pellejero for his extremely valuable bibliographic assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest related to this topic. JB declares advisory/lecture fees and/or travel funding from Amgen, Abbvie, Sanofi-Genzyme, Shire, Vifor-Fresenius-Renal Pharma, and Sanifit. PUT declares advisory/lecture fees and/or travel funding from Abbvie, Amgen, Astellas, Medici, Sanofi, Vifor-Pharma FMC, and Hémotech. MC declares advisory/lecture fees from Amgen, Abbvie, Shire, Vifor-Pharma, and Baxter. CGA declares advisory/lecture fees and/or travel funding from Amgen, Kiowa-Kirin, and FAES.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jordi Bover, Pablo Ureña-Torres and Mario Cozzolino are members of the ERA-EDTA CKD-MBD Working Group.
Rights and permissions
About this article
Cite this article
Bover, J., Ureña-Torres, P., Cozzolino, M. et al. The Non-invasive Diagnosis of Bone Disorders in CKD. Calcif Tissue Int 108, 512–527 (2021). https://doi.org/10.1007/s00223-020-00781-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00781-5