Abstract
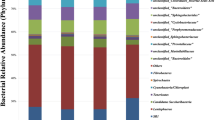
The composition of microorganisms in the gastrointestinal tract is closely related to the intestinal microenvironments and the exterior growth environments of host. In this study, 16S rDNA sequencing technology was adopted to investigate the influence of fermentation bed on the cecum microorganisms of ducks. Two feeding density treatment groups were set up, including group A (n = 4brids/m2) and group B (n = 6brids/m2). Samples were collected from the intermediate core fermentation layer (10–20 cm) of the fermented mattress materials and from the intestinal contents of ducks at 4, 6 and 8 weeks, respectively. Results showed that Bacteroidetes (20.12–27.17%) and Ruminococcaceae UCG-014 (2.97–10.1%) were the predominant microorganisms in duck cecum, while the Truepera (5.08–6.29%), Pricia (4.44–5.44%) and Luteimonas (3.62–4.99%) were the dominant microorganisms in fermentation mattress material. The cecum bacteria exhibited great difference among different growth periods of the ducks. Increasing the stocking density of ducks had a negative effect on the beneficial bacteria in the cecum. The microbial populations in fermentation mattress material were very different from that in the cecal. In summary, our findings can provide a scientific data for the rational use of fermentation bed feeding mode in poultry production.





Similar content being viewed by others
References
Albuquerque L et al (2005) Trueperaradiovictrix gen. nov., sp. Nov., a new radiation resistant species and the proposal of Trueperaceae fam. nov. FEMS Microbiol Lett 247:161–169. https://doi.org/10.1016/j.femsle.2005.05.002
Barnes EM, Mead GC, Barnuml DA, Harry EG (1972) The intestinal flora of the chicken in the period 2 to 6 weeks of age with particular reference to the anaerobic bacteria. Br Poult Sci 13:311–326
Chambers JR, Gong J (2011) The intestinal microbiota and its modulation for Salmonella control in chickens. Food Res Int 44:0–3159
Chen Q, Liu B, Wang J, Che J, Liu G, Guan XJ (2017) Diversity and dynamics of the bacterial community involved in pig manure biodegradation in a microbial fermentation bed system. Ann Microbiol 67:491–500
Chichlowski M, Croom WJ, Edens FW, Mcbride BW, Koci MD (2007) Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, primalac, and salinomycin. Poult Sci 86:1121–1132
Collins SM, Bercik P (2009) The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 136:2003–2014
Costa MC, Bessegatto JA, Alfieri AA, Weese JS, Oba A (2017) Different antibiotic growth promoters induce specific changes in the cecal microbiota membership of broiler chicken. PLoS ONE 12:e0171642
Dezhi WQZDF (2014) Effect of different raising systems on laying performance and egg quality of Beijing you Chicken. China Poult 36:5
Folman LB, Klein Gunnewiek PJ, Boddy L, de Boer W (2008) Impact of white-rot fungi on numbers and community composition of bacteria colonizing beech wood from forest soil. FEMS Microbiol Ecol 63:181–191. https://doi.org/10.1111/j.1574-6941.2007.00425.x
Herve V, Le Roux X, Uroz S, Gelhaye E, Frey-Klett P (2014) Diversity and structure of bacterial communities associated with Phanerochaete chrysosporium during wood decay. Environ Microbiol 16:2238–2252. https://doi.org/10.1111/1462-2920.12347
Ivanova N et al (2011) Complete genome sequence ofTrueperaradiovictrixtype strain (RQ-24T). Stand Genomic Sci 4:91–99
Jha R, Berrocoso JFJAFS (2016) Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim Feed Sci Technol 212:18–26
Jiangrang L et al (2003) Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl Environ Microbiol 69:6816–6824
Józefiak D, Rutkowski A, Jensen BB, Engberg RM (2006) The effect of beta-glucanase supplementation of barley- and oat-based diets on growth performance and fermentation in broiler chicken gastrointestinal tract. Br Poult Sci 47:57–64
Kers JG, Velkers FC, Fischer EAJ, Hermes GDA, Stegeman JA, Smidt H (2018) Host and environmental factors affecting the intestinal microbiota in chickens. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00235
Lagier JC, Armougom F, Mishra AK, Nguyen T-T, Raoult D, Fournier PE (2012) Non-contiguous finished genome sequence and description of Alistipes timonensis sp. nov. Stand Genomic Sci 6(3):315–324
Lifeng Wang JZ, Ma C (2013) Gut microbiota of chickens: a review. Chin J AnimNutr 25:494–502
Lopez-Siles M, Khan TM, Duncan SH, Harmsen HJM, Garcia-Gil LJ, Flint HJ (2012) Cultured representatives of two major phylogroups of human colonic Faecalibacterium prausnitzii can utilize pectin, uronic acids, and host-derived substrates for growth. ASM J 78(2):420–428
Mancabelli L et al (2016) Insights into the biodiversity of the gut microbiota of broiler chickens. Environ Microbiol 18(12):4727–4738
Mantis NJ, Rol N, Corthésy B (2011) Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol 4:603–611
Miller ME et al (2009) Diversity and strain specificity of plant cell wall degrading enzymes revealed by the draft genome of Ruminococcus flavefaciens FD-1. PLoS ONE 4(8):e6650
Oakley BBLH, Kogut MH, Kim WK, Maurer JJ, Pedroso A et al (2014) The chicken gastrointestinal microbiome. FEMS Microbiol Lett 360:100–112
Pan D, Yu Z (2013) Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 5:108–119
Samli HE, Dezcan S, Koc F, Ozduven ML, Okur AA, Senkoylu N (2010) Effects of Enterococcus faecium supplementation and floor type on performance, morphology of erythrocytes and intestinal microbiota in broiler chickens. Br Poult Sci 51:564–568
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60
Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Pallen MJ (2014) Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 9:e91941
She Y, Cai H, Liu G (2018) Effects of antibiotic on microflora in ileum and cecum for broilers by 16S rRNA sequence analysis. Nihon Chikusan Gakkaiho 89:1680–1691
Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ (2014) Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE 8:e84290. https://doi.org/10.1371/journal.pone.0084290
Sufang R, Lihui G, Yanbo L (2013) Effect of fermentation bed on bacterial growth in gastrointestinal tracts of weaned piglets. J Huazhong Agri Univ 32(1):87–91
Teo AY, Tan H-M (2007) Evaluation of the performance and intestinal gut microflora of broilers fed on corn-soy diets supplemented with Bacillus subtilisPB6 (CloSTAT). J Appl Poult Res 16:296–303
Willis WL, Reid L (2008) Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poult Sci 87:606
Xi Y et al (2019) Characteristics of the intestinal flora of specific pathogen free chickens with age. MicrobPathog 132:325–334. https://doi.org/10.1016/j.micpath.2019.05.014
Xiaoting Z, Weijiang Z, Yong L, Jing Y, Wen Y (2015) Study on the change of bacterial community structure of fermented mattress during the breeding of meat duck. Microbiol China 42:1263–1270. https://doi.org/10.13344/j.microbiol.china.140784
Sheng-qiang YE, Ping G, Yu Y et al (2016) Security analysis on duck breeding mode of fermentation bed. Journal of Domestic Animal Ecolog 37(6):95–70
Yegani M, Korver DR (2008) Factors affecting intestinal health in poultry. Poult Sci 87:2052–2063
Zhang C, Li S, Yang L, Huang P, Zhao LJNC (2011) Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat Commun 4:2163
Zheng Xuefang LB, Jianglin L, Bo L, Jianglin L (2011) Study on the biological control effect of microbial fermentation bed on the pathogen of Escherichia coli in pig house. ScientiaAgriculturaSinica 44:4728–4739. https://doi.org/10.3864/j.issn.0578-1752.2011.22.022
Acknowledgements
We are very grateful to this study is funded by the National key Research and Development Program (2018YFD0501500), China Agricultural Research System (CARS-43-6) and Key Technology Support Program of Sichuan Province (2016NYZ0044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
Wang, J.m., Gan, X.m., Pu, Fj. et al. Effect of fermentation bed on bacterial growth in the fermentation mattress material and cecum of ducks. Arch Microbiol 203, 1489–1497 (2021). https://doi.org/10.1007/s00203-020-02145-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02145-x