Abstract
Poor drinking water quality in household wells is hypothesised as being a potential contributor to the high prevalence of chronic kidney disease of uncertain aetiology (CKDu) among the farming communities of the Medawachchiya area, Anuradhapura, Sri Lanka. One of the natural processes that can affect water quality is the dissolution of minerals contained within an aquifer by water–rock interactions (WRIs). Here we present a comprehensive assessment of WRIs and their influence on the water chemistry in household wells and spring waters in the Medawachchiya area by combining measurements of environmental isotopes, such as strontium, lithium and stable carbon isotopes and inorganic chemistry parameters, and modelling geochemical mass balance reactions between rainfall and groundwater samples. Our results reveal the presence of strontium, dissolved from both silicate and carbonate minerals, with high isotopic (87Sr/86Sr) ratios of up to 0.7316. Geochemical mass balance modelling and prior 87Sr/86Sr studies on the Wanni Complex bedrock suggest these strontium values may be the result of biotite dissolution. We also identify lithium and uranium contributed from the dissolution of silicates, albeit at concentrations too low to constitute a known health risk. In contrast, the levels of magnesium and calcium in our samples are high and demonstrate that, despite the felsic bedrock, well water chemistry in the Medawachchiya area is dominated by carbonate dissolution.
Similar content being viewed by others
Introduction
Chronic kidney disease of uncertain aetiology (CKDu) in Sri Lanka was first reported in 1996. The disease currently has a multilevel clinical case definition, whereby disease conditions known to be associated with kidney failure, such as diabetic nephropathy, infectious disease, genetic conditions and others, are excluded1. One hypothesis is that drinking water contaminants may be a causal or contributing factor to the disease2,3,4,5. These include natural drinking water contaminants3,6,7,8, pathogens and microbial toxins9,10,11 and contaminants from pesticides7,12,13,14,15,16,17.
Studies into natural groundwater contaminants in CKDu-affected regions of Sri Lanka have identified higher concentrations of fluoride (F), calcium (Ca) and magnesium (Mg) in Anuradhapura groundwater and lower levels of heavy metals such as cadmium (Cd), arsenic (As) and lead (Pb) compared to the World Health Organisation (WHO) drinking water guideline value and the Sri Lankan potable water standard18,19. Groundwater chemistry in Sri Lanka is largely determined by water–rock interactions (WRIs), ion exchange and precipitation processes20. Natural solutes enter groundwater through dissolution processes, which are enhanced by the input of carbon dioxide, sulfur dioxide and nitrogen oxides into infiltrating rainwater. The concentrations and composition of major ions in groundwater are dependent upon the type of minerals the water interacts with, their resistance to weathering, water residence times and the chemistry of the initial water21. Exchange processes also alter the concentrations of major ions22. Within aquifers, Ca and Mg take the place of adsorbed Na on aquifer solids through cation exchange processes since the interaction between sorption sites and divalent ions is typically much higher than monovalent ions. This results in an increase in dissolved Na concentrations and a decrease in dissolved Ca and Mg concentrations23.
The study of certain environmental isotopes can further enhance the understanding of these WRI processes and provide valuable information on the water source, the types of minerals groundwater has interacted with, the duration of interaction and information on processes, including evaporation, sorption and biological respiration24,25,26,27,28. Strontium isotopic ratios (87Sr/86Sr) in groundwater reflect the 87Sr/86Sr ratios of the source rock, since dissolution and other natural processes do not cause fractionation29,30. The 87Sr/86Sr of carbonates reflects that of Sr contained in the water that they were precipitated from. Over the past 500 million years, the 87Sr/86Sr of seawater has varied approximately from 0.7068 to 0.709231, thus the dissolution of carbonates results in groundwater Sr ratios close to these values32. In igneous rocks, the 87Sr/86Sr ratio is heavily dependent upon the concentration of rubidium (Rb) incorporated into the rock, due to the decay of 87Rb to 87Sr over time33. Old silicate minerals can, therefore, contain high 87Sr/86Sr ratios, with dissolved 87Sr/86Sr values reaching well above seawater ratios34 and up to 0.7625 previously identified in fresh waters35.
Stable lithium isotopes (δ7Li) provide additional information on silicate weathering, as silicate minerals have been identified as the predominant source of Li in waters, even in carbonate-dominated environments36,37. The δ7Li values in continental crust ranges from approximately −5‰ to +5‰38; however, preferential dissolution of 7Li and preferential adsorption and incorporation of 6Li into secondary minerals results in a higher and much broader range of values in river water (+5‰ to +43‰)37,39,40,41 and groundwaters (+7‰ to +37‰)28,42,43. Values as high as +1225‰ have also been reported in groundwater from a peat bog influenced by agrochemicals44.
The aim of this research is to contribute to a more detailed description of the WRI in spring waters and domestic well waters in CKDu-affected regions in order to obtain a better understanding of how specific hydrological and hydrogeochemical factors affecting groundwater might contribute to poor drinking water quality and distinguish them from other non-hydrogeological causes of drinking water contamination. A comprehensive isotopic analysis of the spring water and domestic well waters in CKDu-prevalent regions of Sri Lanka has not yet been undertaken. Here, using 87Sr/86Sr, δ7Li and dissolved inorganic carbon (δ13CDIC) combined with major ion analyses, we show how WRIs affect water quality of 30 domestic well and spring waters used for drinking water purposes in a CKDu-impacted region, Medawachchiya, Sri Lanka.
Results
Geochemistry
Water chemistry data are presented in Supplementary Tables 1 and 2. Well waters were less fresh and contained higher electrical conductivity (EC, between 519 and 2095 μS cm−1, n = 28) than spring waters (between 154 and 467 μS cm−1, n = 2). Average dissolved oxygen (DO) and alkalinity measurements were 3.45 mg L−1 ± 2.60 (1σ) and 376.3 mg L−1 ± 134.39 (1σ), respectively, for the well waters, and 2.4 mg L−1 ± 0.93 (1σ) and 122.8 mg L−1 ± 76.93 (1σ), respectively, for the spring waters. The lowest EC measurement was observed in sample 30 spring water, and the highest EC measured in sample 27 (2096 mg L−1) well water. pH measurements ranged from acidic (4.92) in sample 30 to mildly alkaline (8.06) in sample 27.
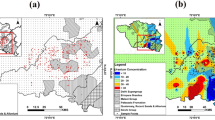
Cluster analysis was used to group the samples for anion and cation data. The analysis shows three distinct clusters of samples (Fig. 1a). Cluster 1 is comprised of 21 samples representing the highest Sr/Cl ratio, Sr/Na ratio and K concentrations, and the lowest Li concentrations (see Supplementary Table 3). Cluster 2 contains 6 samples representing the highest Sr, Cl, Na, Ca and Mg concentrations and lowest Li/Na ratios (Supplementary Table 3). The high concentrations of major ions in samples from Cluster 2 relative to the other cluster groups suggests that these samples may have undergone a higher relative amount of WRI than the other samples. Cluster 3 is comprised of 3 samples representing the lowest Cl, Sr, Na, Ca, Mg, Si and K concentrations and the highest Li concentrations and Li/Na ratios (Supplementary Table 3). This group contains the two spring water samples (samples 13 and 30, Fig. 1a), and likely represent samples that have undergone the least amount of WRI due to the low concentrations of major ions relative to the other groups. These groupings are also confirmed using a principal component analysis (Fig. 1b).
a Cluster dendrogram of samples using Sr/Cl, Sr/Na and Li/Na ratios, major ion concentrations (Cl, Na, Ca, Mg and K), Si, Li and Sr concentrations; b PCA biplot using major ions (Cl, Na, Ca, Mg and K), Sr, Li and Si concentrations in mmol L−1; and c pie charts showing the water types for samples in each cluster group.
All water types are mixed (Fig. 1c), with Cluster group 3 samples notably comprised of waters low in Cl. The dominant anion is HCO3− and the dominant cation is Na. Other ions that contribute significantly to EC in the samples are Cl, SO4, Ca and Mg. There is a clear trend of increasing Ca with increasing EC, with three outliers present (samples 27, 16 and 18) that display reduced Ca relative to EC compared to the other samples (see Supplementary Fig. 1a). These three samples contain high 87Sr/86Sr ratios, high EC, high Na and high U compared to the other samples.
Fluoride concentrations in the Medawachchiya region are high (min = 0.07 mg L−1, max = 3.7 mg L−1, average = 1.2 mg L−1, n = 30) compared to the WHO guideline value for drinking water and the Sri Lankan potable water standard45, with high values in the region also reported in other studies20,46. We observe a trend of increased F concentrations with decreased Ca/Na (rs = −0.60, p = 5.18 × 10–4, n = 30) and Mg/Na ratios (rs = −0.54, p = 1.97 × 10–3, n = 30, see Fig. 1b), suggesting a silicate F source. Reduced Ca/Na and Mg/Na ratios associated with a silicate end-member has been identified in other studies47,48.
The relationship between major ions and Cl are shown in Fig. 2. Most samples contain elevated Na, Mg, SO4 and HCO3 relative to Cl, suggesting a source of these ions in the aquifer. Elevated SO4 relative to Cl suggests the dissolution of gypsum or metamorphic minerals. One sample (sample 01) shows particularly high K relative to the other samples (Fig. 2c) and may indicate input from an anthropogenic source, such as agrochemical application. Group 1 and Group 2 samples are characterised by low K relative to Cl, which may indicate biotic uptake of K. Group 1 displays high Mg (Fig. 2b), HCO3 (Fig. 2f) and several samples with high Ca relative to Cl (Fig. 2d) indicating likely carbonate and/or feldspar dissolution. Two Group 2 samples in the lower right side of Fig. 2d (samples 27 and 16) have the lowest Ca/Cl ratios (0.15 and 0.17, respectively) suggesting carbonate precipitation.
Cl vs a Na, b Mg, c K, d Ca, e SO4 and f HCO3. The black line on each plot indicates the ratio of Cl to each ion present in rainwater from Vavuniya, north of the study site54. Points above the line indicate enrichment in the major ion compared to chloride, suggesting that the source of the ion is from mineral weathering rather than rainwater. Points below the line suggest depletion of the ion compared to Cl, indicating removal through an exchange process or mineral precipitation process.
Groundwater Si concentrations usually range from 1 to 30 mg L−1 49. We identify high Si concentrations in this study, with Si concentrations ranging from 17.0 to 58.4 mg L−1, with an average of 45.1 mg L−1 ± 10.90 (1σ) suggesting that a high level of WRI has taken place in some samples. In the well waters, Sr concentrations ranged from 0.21 to 1.52 mg L−1 (n = 28), while Sr concentrations in the spring waters were comparatively low and ranged from 0.05 to 0.23 mg L−1 (n = 2). The Rb concentrations in all samples were low, between <0.001 and 0.016 mg L−1 for well waters (n = 28) and 0.002 mg L−1 for the two spring water samples. The Li concentrations ranged from 0.002 to 0.016 mg/L in the well waters and from 0.004 to 0.008 mg/L in the two spring waters.
Average 87Sr/86Sr ratios were highest in Group 2 (0.717) and lowest in Group 1 (0.713), with δ7Li (‰) values highest in Group 2 (+18.9‰ ± 6.1) and lowest in Group 3 (+7.3‰ ± 8.6, see Supplementary Table 4). Notably, the lowest and highest δ7Li values correspond with the samples containing the lowest and highest EC values, respectively. The δ13CDIC values ranged from −14.7‰ to −19.9‰ (Supplementary Table 4). The lowest value is found in Group 3 in the freshest spring water sample (sample 30) at −23.5‰, which may indicate DIC originating from an organic source, for example through biodegradation of soil organic matter25.
Geochemical mass balance modelling
The NETPATH computer program was used to interpret net geochemical mass balance reactions between initial and final waters along a user-defined chemical evolution flow path in the aquifer. Geochemical mass balance modelling was used to quantify the amount of carbonate dissolution occurring after rainfall is recharged and forms the observed groundwater sample. Samples 02, 07 and 23 were considered to be the most influenced by carbonate dissolution. It was calculated that between 0.61 and 1.71 mmol kg−1 of calcite was needed to produce the resultant water chemistry, if considering rainwater was the starting water and according to the models in Supplementary Table 5. The model that best predicted the measured δ13CDIC value was sample 23. This model showed that the dissolution of 0.94 mmol kg−1 of calcite, influx of 2.2 mmol kg−1 of CO2, dissolution of 1.3 mmol kg−1 of MgSiO3, addition of 7.4 mmol kg−1 of NaCl from within the aquifer and dissolution of 0.5 mmol kg−1 of hornblende into the rainwater recharge and the removal of 1.9 mmol kg−1 of Ca/Na by exchange and removal of 2.3 mmol kg−1 of SiO2 from the water best describes the hydrochemical evolution of the waters most influenced by carbonate processes.
Mass balance modelling was also used to model samples 16, 18 and 27, which were considered most influenced by silicate weathering processes. Similar starting water, assumptions, constraints and phases were used as describe above. From these models, it was found that higher amounts of calcite (1–2.5 mmol kg−1) were needed to produce the resultant water chemistries. If calcite was not added to the models, the calculated δ13CDIC values were between 3 and 8‰ more depleted than the measured values. The model that best predicted the measured δ13CDIC value for these three samples was sample 18. This model showed that the dissolution of 1.3 mmol kg−1 of calcite, dissolution of 0.14 mmol kg−1 of biotite, influx of 6.0 mmol kg−1 of CO2, addition of 2.4 mmol kg−1 of Ca/Na through exchange, dissolution of 5.8 mmol kg−1 of MgSiO3 and addition of 2.9 mmol kg−1 of NaCl to the rainwater and the removal of 5.5 mmol kg−1 of SiO2 best describes the hydrochemical evolution of silicate influenced waters. Models suggest that all waters in this region are influenced by calcite dissolution.
Discussion
Strontium typically retains the same ratio of 87Sr/86Sr during dissolution into groundwater as its source mineral. The 87Sr/86Sr ratios in minerals are dependent upon the mineral type, age and rubidium content of the rock, as well as the proportion of crustal and mantle sources for igneous rocks and amount of alteration of carbonates and evaporites50. In groundwater, the 87Sr/86Sr ratios represent the relative proportions and 87Sr/86Sr ratios of the dissolved input. For example, where Sr input is 50% from carbonate dissolution where the carbonate 87Sr/86Sr ratio is 0.705 and 50% from silicate dissolution where the 87Sr/86Sr ratio is 0.729, the groundwater 87Sr/86Sr ratio observed will be approximately 0.717 (see Fig. 3). The δ7Li, however, fractionates more readily in groundwater through dissolution and adsorption processes, with 7Li preferentially dissolved51 and 6Li preferentially adsorbed to clay mineral surfaces52. This results in increasingly positive δ7Li values with water-sediment processing (Fig. 3). Strontium isotopes can therefore be used to understand silicate weathering processes, and when used with the δ7Li values, the secondary processes can be defined. For example in sample 30 (the freshest spring water sample), the 87Sr/86Sr ratio of 0.713 is observed, along with a very low δ7Li value for fresh water (−1.8‰). This value could be indicative of 50% Sr from carbonate dissolution and 50% from silicate weathering as well as the dissolution of weathering products containing high amounts of 6Li, such as clay minerals from nearby soils.
The 7Li is preferentially dissolved into groundwater from Li bearing minerals such as granite, with 6Li preferentially adsorbed to mineral and clay surfaces, resulting in increasingly high δ7Li values in groundwater with processing. The 87Sr/86Sr ratios do not fractionate with dissolution, therefore ratios in groundwater are the result of the proportion of Sr from the dissolution of silicates and carbonates.
Figure 4a shows that the increasing Sr concentrations associated with both increasing and decreasing 87Sr/86Sr ratios are likely the result of silicate and carbonate weathering, respectively. Currell and Cartwright53 identified carbonate and silicate weathering in groundwater samples by comparing 87Sr/86Sr ratios and δ13CDIC values. Increasing enrichment in δ13CDIC without an increase in 87Sr/86Sr suggested carbonate input, while increasing 87Sr/86Sr ratios not associated with enrichment in δ13CDIC (‰) indicated silicate inputs. This agrees with our findings which demonstrate that samples trending towards silicate input in Fig. 4a display increased 87Sr/86Sr ratios without an increase in δ13CDIC in Fig. 4b. Figure 4b also shows that samples trending towards carbonate input in Fig. 4a display an increase in δ13CDIC without an increase in 87Sr/86Sr ratios. While carbonates are not expected to comprise a large portion of the geology in the region, they weather more readily with the input of the naturally weakly acidic rainwater, and thus can be a large source of Sr. Dissanayake and Weerasooriya54 report weakly acidic rainwater pH values of <5.5 in the study region, which is supported by the pH value of 4.9 observed in sample 30 from this study. Sample 30 is the freshest samples that has undergone the least amount of WRI as evidenced by its low alkalinity and EC values (41.2 mg L−1 and 154 µS cm−1) relative to the other samples, suggesting that it has not been buffered to a higher pH by carbonate dissolution. Blum et al.24 demonstrated that, in quartz-feldspathic gneiss and granites containing 1% carbonate, 82% of the HCO3 flux was derived from weathering of the carbonates. Similarly, our mass balance modelling results reveal that the samples most influenced by carbonate and silicate weathering, respectively, have all undergone carbonate dissolution processes, with calcite inputs between 0.61 and 2.54 mmol kg−1. This suggests that the differences observed between the two groups are due to the additional weathering of silicates, rather than a lack of carbonate inputs in samples affected by silicate weathering. This could be a result of longer water residence times for samples 27, 16 and 18, allowing for the dissolution of silicates that are more resistant to weathering; however, a groundwater residence time indicator such as tritium would be required to confirm this hypothesis.
a Biplot of 87Sr/86Sr ratio (n = 28) on y-axis with the inverse of Sr concentration (1/Sr (mmol L−1) on the x-axis. Upward and downward trends on the y-axis with increased Sr concentration indicate silicate and carbonate inputs, respectively. b 87Sr/86Sr ratios vs δ13CDIC. Plot highlights that samples displaying increased 87Sr/86Sr with minimal enrichment in δ13CDIC are associated with silicate weathering. Increasing enrichment in δ13CDIC with minimal increases in 87Sr/86Sr are associated with carbonate input. c Biplot with samples trending towards silicate inputs are associated with increased U concentrations. d Biplot of δ7Li on y-axis (n = 21) with the inverse of Li concentration (1/Li (mmol L−1)) on x-axis. Scale bar represents 87Sr/86Sr ratios with dark blue points indicating lower 87Sr/86Sr (carbonate inputs) and higher Li concentrations and lighter blue points indicating higher 87Sr/86Sr (silicate inputs) and lower Li concentrations. NB: no 87Sr/86Sr value available for the grey data point (sample 29) shown to the lower left.
The 87Sr/86Sr ratios of 0.705 have been reported in whole-rock carbonatite samples from the Wanni Complex, with relatively low ratios (0.710) also identified in the felsic fraction of migmatite gneiss in the region55. In contrast, 87Sr/86Sr ratios from biotite in carbonatite rock from the Wanni Complex contain much higher 87Sr/86Sr ratios (approximately 0.729), which our mass transport modelling suggests is likely to be contributing to the high 87Sr/86Sr ratios in samples 21, 6, 24, 18 and 16 as shown in Fig. 4a. These samples also contain higher U concentrations (Fig. 4c) indicating potential hydrolysis of silicate minerals due to carbonic acid to produce soluble uranyl carbonate complexes or assimilation of uranium in the bedrock56. Increasing 87Sr/86Sr ratios as a result of silicate weathering have also been demonstrated in both river catchments33 and groundwaters53. Figure 4d shows a corresponding increase in Li concentration with increasing 87Sr/86Sr ratios (rs = 0.76, p = 3.85 × 10−6) indicating Li input from weathering of silicates.
Dissolution of Li in the absence of adsorption mechanisms would be expected to result in a linear correlation between δ7Li and Li concentration. We do not observe a significant correlation between δ7Li and Li concentration (rs = −0.27, p = 0.22) in the current study, which suggests that there are multiple processes affecting the δ7Li values in groundwater in the Medawachchiya region. Notably, the sample with the highest silicate input and highest 87Sr/86Sr ratio observed in Fig. 4a (sample 16) has the highest δ7Li value and highest EC value but not the highest Li concentration (Fig. 4d), suggesting that Li adsorption may have taken place.
The 87Sr/86Sr ratios found in our study indicate that silicate weathering appears to be the main source of U and Li input in household well waters in the Medawachchiya region. As the water chemistry of most samples in this study appears to be controlled by carbonate dissolution, U and Li concentrations are low (<0.001 mg L−1 for 83% of samples and <0.01 mg L−1 for 77% of samples, respectively).
In contrast, we observe concentrations of F that are higher than guideline values, with 57% of samples over the suggested optimal level of 1.0 mg L−1 of F in Sri Lankan drinking water (SLS614:2013)45. High F levels are known to result in skeletal fluorosis in the dry zone of Sri Lanka57 and are hypothesised to contribute to CKDu58. The significant negative correlation between F and Ca/Na and Mg/Na ratios suggests a silicate source. Experimental data from Chae et al.59 provide evidence that the concentrations of F in groundwater are also highly dependent on Ca concentrations, with increasing Ca inputs resulting in decreasing dissolved F concentrations due to the precipitation of fluorite. We identify high water hardness in the groundwater from the study area, which we propose are not only due to carbonate inputs but also from high silicate weathering, both natural hydrogeochemical processes. We identify only one sample that exceeded the most stringent drinking water limit available of 1.5 mg L−1 Sr. The 1.5 mg L−1 limit is based on the United States Environmental Protection Agency’s preliminary regulatory determinations for contaminants on the third drinking water contaminant candidate list (www.govinfo.gov/app/details/FR-2014-10-20/2014-24582) and is based on safe limits for consumption in sensitive populations such as those with renal failure and other health issues. Our results suggest that U, Li and Sr are unlikely contributors to CKDu due to their low concentrations.
The 87Sr/86Sr, δ7Li and δ13CDIC isotopes enhance the understanding of WRI, which cannot be understood by using major ions alone. This study uses 87Sr/86Sr, δ7Li and δ13CDIC values to demonstrate WRI in drinking water wells in a CKDu-affected region of Sri Lanka. Our study demonstrates that 87Sr/86Sr ratios and δ13CDIC provide clear indication of the input of silicates and carbonates, with carbonate dissolution predominantly controlling the water chemistry in the region. The δ7Li isotopes, in contrast, are more complex and relatively unstudied in groundwater. We conclude that δ7Li data in household well waters in Medawachchiya are impacted by several different processes, which results in a lack of clear correlation between δ7Li and Li concentration. The Li and U input from silicate minerals, however, is observed through the positive correlation between 87Sr/86Sr ratios and Li concentration, and the increase in U concentration in samples with high 87Sr/86Sr ratios. These results highlight the importance of isotopic analyses in determining WRI and sources of solutes in drinking water. While our results suggest that U, Li and Sr are unlikely contributors to CKDu due to their low concentrations, this could be confirmed with time-series analyses to determine the seasonal variability of these parameters.
Materials and methods
Environmental setting
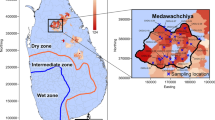
Three main climatic zones are observed in Sri Lanka, defined based on the mean rainfall quantities received per year. These include the “Dry Zone” (<1750 mm year−1), “Intermediate Zone” (1750–2500 mm year−1) and “Wet Zone” (>2500 mm year−1) (Fig. 5). Medawachchiya is in the Dry Zone and receives an average rainfall of 1240 mm year−1, largely in October, November and December where long-term (1960–1985 CE) average monthly rainfall rates are between 206 and 247 mm60. Temperatures are relatively stable throughout the year, with an average minimum monthly temperature of 20.3 °C in January and a maximum average monthly temperature of 34.0 °C in April in Vavuniya60, which is located approximately 22 km north of the Medawachchiya sampling region.
Geology of Sri Lanka showing the Precambrian lithotectonic units: Highland Complex, Wanni Complex, Vijayan Complex, and the Kadugannawa Complex (KC), and the Mesozoic unit to the north-west. Map adapted from Cooray66. Inset shows the sample locations (black diamonds) within the Medawachchiya district (black outline). The geology in the region is predominantly comprised of gneiss including charnockitic gneiss and biotite gneiss, with granitic gneiss, microcline, quartzite and quartz schist67. Samples 1–12 and 14–29 represent well water samples, while samples 13 and 30 represent spring water samples. Dry (<1750 mm year−1), intermediate (1750–2500 mm year−1) and wet (>2500 mm year−1) zones are defined by the blue and orange lines.
The geology in Sri Lanka is mainly comprised of three major high-grade metamorphic rock units: the Highland Complex, the Wanni Complex, and the Vijayan Complex61 (Fig. 5). There are also two smaller units present: the Kadugannawa Complex in the central region of Sri Lanka and a Mesozoic region to the north-west of the island. Medawachchiya is located within the Wanni Complex, which is comprised predominantly of charnockitic (hypersthene) gneiss and charnockitic biotite gneiss, with regions of granitic gneiss, microcline, quartzite and quartz schist. The Wanni Complex contains quartz feldspathic gneiss, which consists of garnet, biotite, potassium (K)-feldspar, plagioclase and quartz. The charnockite contains orthopyroxene, garnet, hornblende, biotite, K-feldspar, plagioclase and quartz62.
Sampling
A total of 30 domestic well water and spring water samples were collected in Medawachchiya, Sri Lanka (Fig. 5). Within this CKDu-endemic region, we aimed to sample a range of well waters that we suspected to have undergone different WRIs. These included groundwaters along a shear zone (samples 1, 2, 3, 16), groundwaters along the axis of an antiform (samples 7, 11, 15, 17, 23) and spring water samples (samples 13 and 30). Depth to the water table ranged from 0.0 m to 9.0 m below ground surface, with samples obtained from 1 m below the standing water level using a peristaltic pump. Samples were filtered using a high flow Waterra 0.45 µm filter connected to the pump outlet, with sample bottles filled after rinsing three times with the water to be sampled. Tape was then placed around the bottle lids to prevent leakage and gas exchange. Cations and trace element samples were collected in 60 and 250 ml high-density polyethylene (HDPE) bottles, respectively, with 0.4 and 0.9 ml of ultrapure HNO3 added, respectively, and refrigerated. Anions and stable water isotopes were collected in 60 and 30 ml HDPE bottles, respectively. The δ13CDIC, 87Sr/86Sr and δ7Li samples were collected in 12 ml glass vials, 60 ml HDPE and 250 ml HDPE pretreated bottles, respectively.
Analysis
Field parameters including water temperature, pH, DO and EC were monitored using a flow cell connected to a YSI meter to ensure that parameters were stable prior to sampling, with values recorded in the field at the time of sampling. Anions were analysed at ANSTO using ion chromatography and cations were analysed at ANSTO using inductively coupled plasma-atomic emission spectroscopy and inductively coupled plasma mass spectrometry.
The δ7Li samples were analysed in a Class 100 cleanroom at the University of Wollongong Isotope Geochronology Laboratory using a Thermo Fisher Neptune Plus multi-collector inductively coupled plasma mass spectrometer. Samples were prepared by drying down an aliquot of sample containing ~60 ng of Li and adding 0.5 mL 1 M HCl. A two-step cation exchange chromatography procedure was used to collect pure Li. In all, 30 mL Savillex micro columns were used with Biorad AG50W-X8 resin as a cation exchange medium. Columns were cleaned with 30 mL of 6 M HCl, rinsed with ultrapure water and conditioned with 1 M HCl before sample loading. After the sample was passed through the exchange columns once, the sample was dried on a hotplate, 1 M HCl was added and the sample was reloaded into the column a second time. The sample was then dried again on a hotplate and digested in 0.3 M HNO3 prior to isotopic analysis. A 30 parts per billion solution of isotopic standard IRMM-16 was used to correct the 7Li/6Li values for mass bias, with the results of the instrument blank used to correct each isotope for standard and sample analyses. Results were then converted to δ7Li, with isotopic ratios normalised using L-SVEC (Eq. 1) from Carignan et al.63:
Error values for δ7Li values did not exceed 0.20‰ (min = 0.07‰, average = 0.10‰, σ = 0.03‰).
The 87Sr/86Sr isotopes were analysed at the Australian National University Research School of Earth Sciences using ion-specific resin chromatography followed by thermal ionisation mass spectrometry. International strontium carbonate isotopic standard NBS987 was run 6 times during analysis of the samples, with an average 87Sr/86Sr ratio of 0.710262 measured and a standard deviation (σ) of 0.000005. The standard errors of the samples did not exceed 0.000011. The δ13CDIC were analysed at ANSTO using a continuous-flow Delta V Advantage isotope ratio mass spectrometer as per the methods published in Assayag et al.64. Results were reported as ‰ deviations from the NBS19 international carbonate standard.
Geochemical mass balance modelling
To evaluate the validity of the proposed hydrochemical processes, net transfer models65 were applied to well water samples.
Hydrogeochemical models were constructed using C, Mg, Cl, Ca, Na, redox and Si as constraints. The modelled phases were organic matter (CH2O), CO2(g), hornblende, Mg/Na Ex, MgSiO3, SiO2, NaCl (from atmospheric sources), biotite, Ca/Na exchange and calcite. The pH and major ion (Ca, Mg, Na, K, Cl and SO4) concentrations from a rainwater sample from Vavuniya, also located within the Dry Zone approximately 23 km north of the study area, was used as the initial water54. The system is assumed to be receiving piston flow recharge and the groundwater system is in chemical equilibrium. Furthermore, carbon mass balance using Rayleigh distillation equations for all incoming carbon sources and all isotopically fractionating outgoing carbon phases were calculated65. Models were assessed for accuracy by comparing the observed and computed δ13CDIC results produced by each model and most fell within ±2‰ for the difference between modelled and measured δ13CDIC values. Models that failed to predict the δ13CDIC value were not used in the interpretation.
Data availability
The full data set used in this study is provided in Supplementary Information.
Code availability
The code used to develop individual figures is available upon request to the corresponding author.
References
Wijewickrama, E. S., Gunawardena, N., Jayasinghe, S. & Herath, C. CKD of unknown etiology (CKDu) in Sri Lanka: a multilevel clinical case definition for surveillance and epidemiological studies. Kidney Int. Rep. 4, 781–785 (2019).
Balasooriya, S. et al. Possible links between groundwater geochemistry and chronic kidney disease of unknown etiology (CKDu): an investigation from the Ginnoruwa region in Sri Lanka. Expos. Health. https://doi.org/10.1007/s12403-019-00340-w (2019).
Chandrajith, R. et al. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environ. Geochem. Health 33, 267–278 (2011).
Dharma-wardana, M. W. C., Amarasiri, S. L., Dharmawardene, N. & Panabokke, C. R. Chronic kidney disease of unknown aetiology and ground-water ionicity: study based on Sri Lanka. Environ. Geochem. Health 37, 221–231 (2015).
Edirisinghe, E. A. N. V., Manthrithilake, H., Pitawala, H. M. T. G. A., Dharmagunawardhane, H. A. & Wijayawardane, R. L. Geochemical and isotopic evidences from groundwater and surface water for understanding of natural contamination in chronic kidney disease of unknown etiology (CKDu) endemic zones in Sri Lanka. Isotopes Environ. Health Stud. 54, 244–261 (2017).
Dissanayake, C. B. & Chandrajith, R. Groundwater fluoride as a geochemical marker in the etiology of chronic kidney disease of unknown origin in Sri Lanka. Ceylon J. Sci. 46, 3–12 (2017).
Jayasumana, M. A. C. S. et al. Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J. Nat. Sci. Res. 3, 64–73 (2013).
Wasana, H. M. S. et al. Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environ. Geochem. Health 38, 157–168 (2016).
Desalegn, B. et al. Mycotoxin detection in urine samples from patients with chronic kidney disease of uncertain etiology in Sri Lanka. Bull. Environ. Contamination Toxicol. 87, 6–10 (2011).
Manage, P. M. Cyanotoxins: a hidden cause of chronic kidney disease of unknown etiology (CKDu) in Sri Lanka-a review. Sri Lanka J. Aquat. Sci. 24, 1–23 (2019).
McDonough, L. K. et al. The water chemistry and microbiome of household wells in Medawachchiya, Sri Lanka, an area with high prevalence of chronic kidney disease of unknown origin (CKDu). Sci. Rep. 10, 18295 (2020).
Chandrajith, R. et al. Natural radionuclides and trace elements in rice field soils in relation to fertilizer application: study of a chronic kidney disease area in Sri Lanka. Environ. Earth Sci. 60, 193–201 (2010).
Diyabalanage, S. et al. Has irrigated water from Mahaweli River contributed to the kidney disease of uncertain etiology in the dry zone of Sri Lanka? Environ. Geochem. Health 38, 679–690 (2016).
Jayasumana, C. et al. Phosphate fertilizer is a main source of arsenic in areas affected with chronic kidney disease of unknown etiology in Sri Lanka. SpringerPlus 4, 90 (2015).
Jayasumana, C., Gunatilake, S. & Siribaddana, S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 16, 103 (2015).
Jayasumana, C., Gajanayake, R. & Siribaddana, S. Importance of Arsenic and pesticides in epidemic chronic kidney disease in Sri Lanka. BMC Nephrol. 15, 124 (2014).
Wimalawansa, S. J. Agrochemicals and chronic kidney disease of multi-factorial origin: environmentally induced occupational exposure disease. Int. J. Nephrol. Kidney Fail. https://doi.org/10.16966/2380-5498.111 (2015).
Herath, H. M. A. S. et al. Potential risk of drinking water to human health in Sri Lanka. Environ. Forensics 18, 241–250 (2017).
Wickramarathna, S., Balasooriya, S., Diyabalanage, S. & Chandrajith, R. Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka—a hydrogeochemical and isotope approach. J. Trace Elem. Med. Biol. 44, 298–306 (2017).
Cooray, T. et al. Assessment of groundwater quality in CKDu affected areas of Sri Lanka: implications for drinking water treatment. Int. J. Environ. Res. Public Health 16, 1698 (2019).
Elango, L. & Kannan, R. in Developments in Environmental Science, Vol. 5 (eds. Sarkar, D., Datta, R. & Hannigan, R.) 229–243 (Elsevier, 2007).
Putnis, A. Mineral replacement reactions: from macroscopic observations to microscopic mechanisms. Mineralogical Mag. 66, 689–708 (2002).
Hem, J. D. Study and Interpretation of the Chemical Characteristics of Natural Water. Report No. 9781410223081 (U.S. Geological Survey, 1985).
Blum, J. D., Gazis, C. A. & Jacobson, A. D. & Chamberlain, C. P. Carbonate versus silicate weathering in the Raikhot watershed within the High Himalayan Crystalline Series. Geology 26, 411–414 (1998).
Campeau, A. et al. Multiple sources and sinks of dissolved inorganic carbon across Swedish streams, refocusing the lens of stable C isotopes. Sci. Rep. 7, 9158 (2017).
Edirisinghe, E. A. N. V., Pitawala, H. M. T. G. A., Dharmagunawardhane, H. A. & Wijayawardane, R. L. Spatial and temporal variation in the stable isotope composition (δ18O and δ2H) of rain across the tropical island of Sri Lanka. Isotopes Environ. Health Stud. 53, 628–645 (2017).
Hu, D. et al. 87Sr/86Sr evidence from the epeiric Martin Ridge Basin for enhanced carbonate weathering during the Hirnantian. Sci. Rep. 7, 11348 (2017).
Martin, A. N., Meredith, K., Norman, M. D., Bryan, E. & Baker, A. Lithium and strontium isotope dynamics in a carbonate island aquifer, Rottnest Island, Western Australia. Sci. Total Environ. 715, 136906 (2020).
Flockhart, D. T. T., Kyser, T. K., Chipley, D., Miller, N. G. & Norris, D. R. Experimental evidence shows no fractionation of strontium isotopes (87Sr/86Sr) among soil, plants, and herbivores: implications for tracking wildlife and forensic science. Isotopes Environ. Health Stud. 51, 372–381 (2015).
Graustein, W. C. in Stable Isotopes in Ecological Research (eds Rundel, P. W., Ehleringer, J. R. & Nagy, K. A.) 491–512 (Springer, New York, 1989).
Veizer, J. Strontium isotopes in seawater through time. Annu. Rev. Earth Planet. Sci. 17, 141–167 (1989).
Cartwright, I., Weaver, T., Cendón, D. I. & Swane, I. Environmental isotopes as indicators of inter-aquifer mixing, Wimmera region, Murray Basin, Southeast Australia. Chem. Geol. 277, 214–226 (2010).
Shand, P., Darbyshire, D. P. F., Love, A. J. & Edmunds, W. M. Sr isotopes in natural waters: applications to source characterisation and water–rock interaction in contrasting landscapes. Appl. Geochem. 24, 574–586 (2009).
Meredith, K. T. et al. Evolution of dissolved inorganic carbon in groundwater recharged by cyclones and groundwater age estimations using the 14C statistical approach. Geochim. Cosmochim. Acta 220, 483–498 (2018).
Harrington, G. A. & Herczeg, A. L. The importance of silicate weathering of a sedimentary aquifer in arid Central Australia indicated by very high 87Sr/86Sr ratios. Chem. Geol. 199, 281–292 (2003).
Kısakűrek, B., James, R. H. & Harris, N. B. W. Li and δ7Li in Himalayan rivers: proxies for silicate weathering? Earth Planet. Sci. Lett. 237, 387–401 (2005).
Wang, Q.-L. et al. Behavior of lithium isotopes in the Changjiang River system: sources effects and response to weathering and erosion. Geochim. Cosmochim. Acta 151, 117–132 (2015).
Teng, F. Z. et al. Lithium isotopic composition and concentration of the upper continental crust. Geochim. Cosmochim. Acta 68, 4167–4178 (2004).
Huh, Y., Chan, L.-H., Zhang, L. & Edmond, J. M. Lithium and its isotopes in major world rivers: implications for weathering and the oceanic budget. Geochim. Cosmochim. Acta 62, 2039–2051 (1998).
Liu, C.-Q., Zhao, Z.-Q., Wang, Q. & Gao, B. Isotope compositions of dissolved lithium in the rivers Jinshajiang, Lancangjiang, and Nujiang: implications for weathering in Qinghai-Tibet Plateau. Appl. Geochem. 26, S357–S359 (2011).
Millot, R., Vigier, N. & Gaillardet, J. Behaviour of lithium and its isotopes during weathering in the Mackenzie Basin, Canada. Geochim. Cosmochim. Acta 74, 3897–3912 (2010).
Tomascak, P. B. Developments in the understanding and application of lithium isotopes in the earth and planetary sciences. Rev. Mineral. Geochem. 55, 153–195 (2004).
Meredith, K., Moriguti, T., Tomascak, P., Hollins, S. & Nakamura, E. The lithium, boron and strontium isotopic systematics of groundwaters from an arid aquifer system: implications for recharge and weathering processes. Geochim. Cosmochim. Acta 112, 20–31 (2013).
Négrel, P., Millot, R., Brenot, A. & Bertin, C. Lithium isotopes as tracers of groundwater circulation in a peat land. Chem. Geol. 276, 119–127 (2010).
Sri Lanka Standards Institution. Sri Lanka Standards Institution Specification for Potable Water: 2013. First Revision (Sri Lanka Standards Institution, Colombo, 2013).
Chandrajith, R., Dissanayake, C. B., Ariyarathna, T., Herath, H. M. J. M. K. & Padmasiri, J. P. Dose-dependent Na and Ca in fluoride-rich drinking water—another major cause of chronic renal failure in tropical arid regions. Sci. Total Environ. 409, 671–675 (2011).
Torres, M. A., Moosdorf, N., Hartmann, J., Adkins, J. F. & West, A. J. Glacial weathering, sulfide oxidation, and global carbon cycle feedbacks. Proc. Natl Acad. Sci. USA 114, 8716–8721 (2017).
Kumar, S., Singh, R., Venkatesh, A. S., Udayabhanu, G. & Sahoo, P. R. Medical geological assessment of fluoride contaminated groundwater in parts of Indo-Gangetic Alluvial plains. Sci. Rep. 9, 16243 (2019).
Haines, T. S. & Lloyd, J. W. Controls on silica in groundwater environments in the United Kingdom. J. Hydrol. 81, 277–295 (1985).
Brems, D. et al. Isotopes on the beach, part 1: strontium isotope ratios as a provenance indicator for lime raw materials used in Roman glass-making. Archaeometry 55, 214–234 (2013).
Taylor, T. I. & Urey, H. C. Fractionation of the lithium and potassium isotopes by chemical exchange with zeolites. J. Chem. Phys. 6, 429–438 (1938).
Garcia, M. G., Borda, L. G., Godfrey, L. V., López Steinmetz, R. L. & Losada-Calderon, A. Characterization of lithium cycling in the Salar De Olaroz, Central Andes, using a geochemical and isotopic approach. Chem. Geol. 531, 119340 (2020).
Currell, M. J. & Cartwright, I. Major-ion chemistry, δ13C and 87Sr/86Sr as indicators of hydrochemical evolution and sources of salinity in groundwater in the Yuncheng Basin, China. Hydrogeol. J. 19, 835 (2011).
Dissanayake, C. B. & Weerasooriya, S. V. R. The environmental chemistry of rainwater in Sri Lanka. Int. J. Environ. Stud. 26, 71–86 (1985).
Weerakoon, M. W. K., Miyazaki, T., Shuto, K. & Kagami, H. Rb-Sr and Sm-Nd geochronology of the Eppawala metamorphic rocks and carbonatite, Wanni Complex, Sri Lanka. Gondwana Res. 4, 409–420 (2001).
Moon, S. H. et al. Establishing the origin of elevated uranium concentrations in groundwater near the Central Ogcheon Metamorphic Belt, Korea. J. Environ. Qual. 42, 118–128 (2013).
Dissanayake, C. B. The fluoride problem in the ground water of Sri Lanka—environmental management and health. Int. J. Environ. Stud. 38, 137–155 (1991).
Wimalawansa, S. J. Does fluoride cause the mysterious chronic kidney disease of multifactorial origin? Environ. Geochem. Health 42, 3035–3057 (2020).
Chae, G.-T., Yun, S.-T., Kwon, M.-J., Kim, Y.-S. & Mayer, B. Batch dissolution of granite and biotite in water: Implication for fluorine geochemistry in groundwater. Geochem. J. 40, 95–102 (2006).
Pathmarajah, S. Groundwater Availability and Use in the Dry Zone of Sri Lanka (Cap-Net Lanka, PGIA, Peradeniya, 2016).
Dissanayake, C. B. & Chandrajith, R. Sri Lanka–Madagascar Gondwana linkage: evidence for a pan‐African mineral belt. J. Geol. 107, 223–235 (1999).
Kitano, I., Osanai, Y., Nakano, N., Adachi, T. & Fitzsimons, I. C. W. Detrital zircon and igneous protolith ages of high-grade metamorphic rocks in the Highland and Wanni Complexes, Sri Lanka: their geochronological correlation with southern India and East Antarctica. J. Asian Earth Sci. 156, 122–144 (2018).
Carignan, J., Vigier, N. & Millot, R. Three secondary reference materials for lithium isotope measurements: Li7-N, Li6-N and LiCl-N solutions. Geostand. Geoanal. Res. 31, 7–12 (2007).
Assayag, N., Rivé, K., Ader, M., Jézéquel, D. & Agrinier, P. Improved method for isotopic and quantitative analysis of dissolved inorganic carbon in natural water samples. Rapid Commun. Mass Spectrom. 20, 2243–2251 (2006).
Plummer, L., Prestemon, E. & Parkhurst, D. NETPATH-An Interactive Code (NETPATH) For Modeling Net Geochemical Reactions along a Flow Path (U.S. Geological Survey, Reston, Virginia, 1994).
Cooray, P. G. The Precambrian of Sri Lanka: a historical review. Precambrian Res. 66, 3–18 (1994).
Cooray, P. G. Geological Map of Sri-Lanka. Available at: https://esdac.jrc.ec.europa.eu/content/geological-map-sri-lanka. (King Abdul Aziz University, Jiddah, Kingdom of Saudi Arabia, 1982).
Acknowledgements
The authors would like to thank Rohana Chandrajith from the University of Peradeniya for various support. We would also like to thank Asanga Rayasinhe from the Sri Lankan Ministry of Health, Tharanga Senevirathna and Lakruwan Alwis from the National Water Supply and Drainage Boards and Mahesh Karunarathne and Dilki Ekanayake from the University of Peradeniya for their assistance in field sampling. We thank various ANSTO personnel including Barbora Gallagher for stable water isotope and 13CDIC analyses, Henri Wong for anion/cation analyses and Chris Dimovski for assistance with fieldwork preparation and sample organisation. We also thank Adi Paterson and Suzanne Hollins for discussions in the field. Thanks to the other project team members: Frederic Sierro, Ryan Middleton, Katrina Van de Ven and Marina Sara for project management, guidance and ideas. We thank Marc Norman from Australian National University for strontium isotope analyses and Anthony Dosseto and Holly Taylor from University of Wollongong for lithium isotope analyses. This project was funded by the Australian Department of Foreign Affairs and Trade and ANSTO.
Author information
Authors and Affiliations
Contributions
Sampling design and the suite of water quality analyses were organised by C.N. and K.T.M. Field sampling was undertaken by C.N. and R.B.B. L.K.M. drafted the main manuscript, undertook statistical associations between water quality parameters and environmental isotopes and prepared figures under the guidance of K.T.M. C.N. provided input regarding the analysis of water chemistry data and sample site details. All authors reviewed and provided comments on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McDonough, L.K., Meredith, K.T., Nikagolla, C. et al. The influence of water–rock interactions on household well water in an area of high prevalence chronic kidney disease of unknown aetiology (CKDu). npj Clean Water 4, 2 (2021). https://doi.org/10.1038/s41545-020-00092-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-020-00092-0
This article is cited by
-
Molecular Linkage of Dissolved Organic Matter in Groundwater with Prevalence of Chronic Kidney Disease with Unknown Etiology
Exposure and Health (2023)
-
Hydrogeochemical factors controlling the occurrence of chronic kidney disease of unknown etiology (CKDu)
Environmental Geochemistry and Health (2023)
-
Recognizing the groundwater related to chronic kidney disease of unknown etiology by humic-like organic matter
npj Clean Water (2022)
-
Hydrochemical and isotopic baselines for understanding hydrological processes across Macquarie Island
Scientific Reports (2022)