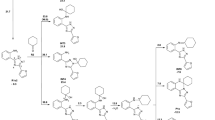
The reaction of substituted 4,4-diphenyl-3,4(1,4)-dihydroquinazolines with various alkylating agents in DMSO–KOH leads (depending on the structure of the alkylating agent) to the formation of the corresponding N1-monoalkyl-substituted 1,4-dihydroquinazolines or, in the reaction with ethyl bromoacetate, to N-{[5-bromo-2-(methylamino)phenyl]diphenylmethyl}benzamide with opening of the heterocyclic ring. The molecular structures of 1-ethyl-2,4,4-triphenyl-1,4-dihydroquinazoline and 1-ethyl-2-(7-methoxy-1,3-benzodioxol-5-yl)-4,4-diphenyl-1,4-dihydroquinazoline were investigated and proven by X-ray structural analysis.


Similar content being viewed by others
References
Gromachevskaya, E. V.; Kaigorodova, E. A.; Konyushkin, L. D.; Krapivin, G. D. Chem. Heterocycl. Compd. 2018, 54, 887. [Khim. Geterotsikl. Soedin. 2018, 54, 887.]
Gromachevskaya, E. V.; Kaigorodova, E. A.; Konyushkin, L. D. Chem. Heterocycl. Compd. 2017, 53, 545. [Khim. Geterotsikl. Soedin. 2017, 53, 545.]
Gromachevskaya, E. V.; Kaigorodova, E. A.; Pushkareva, K. S.; Krapivin, G. D. Chem. Heterocycl. Compd. 2013, 48, 1492. [Khim. Geterotsikl. Soedin. 2012, 1603.]
Gromachevskaya, E. V.; Kaigorodova, E. A.; Firgang S. I.; Krapivin, G. D. Chem. Heterocycl. Compd. 2005, 41, 1045. [Khim. Geterotsikl. Soedin. 2005, 1222.]
Gromachevskaya, E. V.; Krapivin, G. D.; Kvitkovsky, F. V.; Shein, A. O.; Kul'nevitch, V. G. Chem. Heterocycl. Compd. 2001, 37, 588. [Khim. Geterotsikl. Soedin. 2001, 640.]
Vandyck, K.; Verschueren, W. G.; Raboisson, P. J.-M. B. US Patent 9126986.
Pfrengle, W.; Frank, M.; Klein, T. US Patent 8962636.
Zhang, J.; Zhang, Y.; Xie, H.; Ren, Q.; Hu, B.; Fu, C.; Wu, X.; Li, S.; Wang, C.; Zhang, Z. WO Patent 2014082379.
Vandyck, K.; Verschueren, W. G.; Raboisson, P. J.-M. B. WO Patent 2013098313.
Pfrengle, W.; Frank, M.; Klein, T. WO Patent 2013010964.
Vandyck, K.; Verschueren, W. G.; Raboisson, P. J.-M. B. WO Patent 2012013643.
Sherlock, M. H. US Patent 3466284.
Orzalesi, G.; Selleri, R.; Landi Vittory, R.; Innocenti, F.; Grandolini, G. J. Heterocycl. Chem. 1977, 14, 733.
Cox, D. A. US Patent 3631035.
Menteşe, E.; Karaali, N.; Akyüz, G.; Yılmaz, F.; Ülker, S.; Kahveci, B. Chem. Heterocycl. Compd. 2016, 52, 1017. [Khim. Geterotsikl. Soedin. 2016, 52, 1017.]
Sedenkova, K. N.; Averina, E. B.; Nazarova, A. A.; Grishin, Yu. K.; Karlov, D. S.; Zamoyski, V. L.; Grigoriev, V. V.; Kuznetsova, T. S.; Palyulin, V. A. Mendeleev Commun. 2018, 28, 423.
Gromachevskaya, E. V.; Fin'ko, A. V.; Butin, A. V.; Pushkareva, K. S.; Strelkov, V. D.; Isakova, L. I.; Krapivin, G. D. Chem. Heterocycl. Compd. 2013, 49, 1331. [Khim. Geterotsikl. Soedin. 2013, 1428.]
Gormachevskaya, E. V.; Kaigorodova, E. A.; Strelkov, V. D.; Balakhov, А. А.; Lysenko S. A.; Krapivin G. D. RF Patent 2574067; Byull. Izobret. 2016, (4); Chem. Abstr. 2016, 164, 225864.
Kul'nevich, V. G.; Gromachevskaya, E. V.; Kosulina, T. P. Chem. Heterocycl. Compd. 1984, 20, 776. [Khim. Geterotsikl. Soedin. 1984, 953.]
Aroney, M. I.; Le Fevre, R. J. W.; Ritchie, G. L. D.; Singh, A. N. J. Chem. Soc. 1965, 5810
Korshak, V. V.; Kolodyazhnyi, Yu. V.; Vasnev, V. A.; Vinogradov, S. V.; Tsapkova, N. N.; Danashvili, М. М.; Bulgarevich, S. B.; Sadimenko, A. P. Dokl. Akad. Nauk SSSR 1980, 253, 1140.
Andose, J. D.; Mislow, K. J. J. Am. Chem. Soc. 1974, 96, 2168.
Riche, C.; Pascard-Billy, C. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1974, B30, 1874.
Butin, A. V.; Krapivin, G. D.; Zavodnik, V. E.; Kul'nevich, V. G. Chem. Heterocycl. Compd. 1996, 32, 555. [Khim. Geterotsikl. Soedin. 1996, 648.]
CrysAlisPro, version 1.171.39.46; Rigaku Oxford Diffraction, 2018.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, A71, 3.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(12), 1548–1553
*For Communication 7, see 1.
Rights and permissions
About this article
Cite this article
Gromachevskava, E.V., Kaigorogova, E.A., Bespalov, A.V. et al. Investigation in the field of quinazolines. 8*. New reaction of N-alkylation of 4,4-diphenyl-3,4-dihydroquinazolines. Chem Heterocycl Comp 56, 1548–1553 (2020). https://doi.org/10.1007/s10593-020-02848-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02848-5