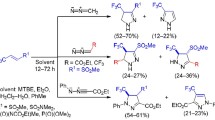
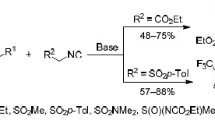
The reactions of 2-pyridinesulfenyl halides with cyclopentene, 1,4-cyclohexadiene, 1,5-cyclooctadiene, 1,3-cyclooctadiene, and norbornene, depending on the structure of the alkene, the nature of the halogen, and the duration of the process, lead to the formation of two types of adducts, electrophilic addition products or condensed compounds, [1,3]thiazolo[3,2-a]pyridinium derivatives, in high yields.
Similar content being viewed by others
References
(a) Pozharskii, A. F.; Soldatenkov, A.; Katritzky, A. R. Heterocycles in Life and Society: an Introduction to Heterocyclic Chemistry, Biochemistry and Applications; John Wiley and Sons: Chichester, 2011, 2nd d. (b) Li, J. J. Heterocyclic Chemistry in Drug Discovery; John Wiley & Sons: Chichester, 2013. (c) Lukevits, E. Chem. Heterocycl. Compd. 1995, 31, 639. [Khim. Geterotsikl. Soedin. 1995, 723.] (d) Gomtsyan, A. Chem. Heterocycl. Compd. 2012, 48, 7. [Khim. Geterotsikl. Soedin. 2012, 12.]
(a) Manfroni, G.; Meschini, F. M.; Barreca, L.; Leyssen, P.; Samuele, A.; Iraci, N.; Sabatini, S.; Massari, S.; Maga, G.; Neyts, J.; Cecchetti, V. Bioorg. Med. Chem. 2012, 20, 866. (b) Khalifa, N. M.; Adel, A. H.; Abd-Elmoez, S. I.; Fathalla, O. A.; El-Gwaad, A. A. A. Res. Chem. Intermed. 2015, 41, 2295. (c) Haddach, M.; Schwaebe, M. K.; Michaux, J.; Nagasawa, J.; O’Brien, S. E.; Whitten, J. P.; Pierre, F.; Kerdoncuff, P.; Darjania, L.; Stansfield, R.; Drygin, D.; Anderes, K.; Proffitt, C.; Bliesath, J.; Siddiqui-Jain, A.; Omori, M.; Huser, N.; Rice, W. G.; Ryckman, D. M. ACS Med. Chem. Lett. 2012, 3, 602.
(a) Shi, F.; Li, C.; Xia, M.; Miao, K.; Zhao, Y.; Tu, S.; Zheng, W.; Zhang, G.; Ma, N. Bioorg. Med. Chem. Lett. 2009, 19, 5565. (b) Vanessa, G.; Sidnei, M.; Alex, F.; Darlene, F.; Pio, C.; Ernani, P. J. Braz. Chem. Soc. 2010, 21, 8. (c) Walker, K. A.; Sjogren, E. B.; Matthews, T. R. J. Med. Chem. 1985, 28, 1673.
(a) Good, J. A. D.; Kulen, A. M.; Almqvist, K. F.; Cairns, A. G.; Ponten, J. F. WO Patent 2016075296; Chem. Abstr. 2016, 164, 602146. (b) El-Hag Ali, G.; Khalil, A.; Lamphon, R.; El-Maghraby, A. Phosphorus, Sulfur Silicon Relat. Elem. 2005, 180, 1909. (c) El-Maghraby, A.; El-Hag Ali, A.; Ahmed, G. A. M.; El-Gaby, A. H. A. Phosphorus, Sulfur Silicon Relat. Elem. 2002, 177, 293.
(a) Potapov, V. A.; Musalov, M. V.; Amosova, S. V. Tetrahedron Lett. 2011, 52, 4606. (b) Musalov, M. V.; Yakimov, V. A.; Potapov, V. A.; Amosova, S. V.; Borodina, T. N.; Zinchenko, S. V. New J. Chem. 2019, 43, 18476.
(a) Potapov, V. A.; Malinovich, D. A.; Amosova, S. V.; Rusakov, Yu. Yu.; Bhasin, K. K. Chem. Heterocycl. Compd. 2012, 48, 1129. [Khim. Geterotsikl. Soedin. 2012, 1208.] (b) Ishigeev, R. S.; Potapov, V. A.; Amosova, S. V. Russ. J. Org. Chem. 2018, 54, 1262. [Zh. Org. Khim. 2018, 54, 1248.] (c) Potapov, V. A.; Ishigeev, R. S.; Amosova, S. V. Russ. J. Org. Chem. 2017, 53, 1604. [Zh. Org. Khim. 2017, 53, 1568.] (d) Potapov, V. A.; Musalova, M. V.; Ishigeev, R. S.; Musalov, M. V.; Panov, V. A.; Khabibulina, A. G.; Amosova, S. V.; Bhasin, K. K. Tetrahedron Lett. 2016, 57, 5341.
(a) Potapov, V. A.; Ishigeev, R. S.; Shkurchenko, I. V.; Zinchenko, S. V.; Amosova, S. V. Molecules 2020, 25, 376. (b) Potapov, V. A.; Ishigeev, R. S.; Shkurchenko, I. V.; Amosova, S. V. Russ. J. Org. Chem. 2019, 89, 2601. [Zh. Org. Khim. 2019, 89, 1965.]
Potapov, V. A.; Ishigeev, R. S.; Amosova, S. V.; Borodina, T. N. Tetrahedron Lett. 2019, 60, 475.
(a) Potapov, V. A.; Ishigeev, R. S.; Amosova, S. V. Russ. J. Org. Chem. 2016, 52, 918. [Zh. Org. Khim. 2016, 52, 922.] (b) Ishigeev, R. S.; Potapov, V. A.; Amosova, S. V. Russ. J. Org. Chem. 2018, 54, 1262. [Zh. Org. Khim. 2018, 54, 1248.] (c) Potapov, V. A.; Ishigeev, R. S.; Amosova, S. V.; Zinchenko, S. V. Russ. Chem. Bull., Int. Ed. 2018, 67, 2326. [Изв. АН, Сер. хим. 2018, 2326.]
(a) Borisov, A. V.; Osmanov, V. K.; Borisova, G. N.; Matsulevich, Zh. V.; Fukin, G. K. Mendeleev Commun. 2009, 19, 49. (b) Borisov, A. V.; Matsulevich, Zh. V.; Osmanov, V. K.; Borisova, G. N.; Mammadova, G. Z.; Maharramov, A. M.; Khrustalev, V. N. Chem. Heterocycl. Compd. 2012, 48, 1098. [Khim. Geterotsikl. Soedin. 2012, 1180.]
This work was supported by the Russian Science Foundation (project 18-13-00372).
Spectral studies were carried out using the material and technical base of the center for collective use “Baikal Analytical Center” of the Siberian Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(11), 1586–1591
Rights and permissions
About this article
Cite this article
Ishigeev, R.S., Potapov, V.А., Shkurchenko, I.V. et al. Two types of products in the reactions of 2-pyridinesulfenyl halides with cycloalkenes and cycloalkadienes: synthesis of novel [1,3]thiazolo[3,2-a]pyridinium derivatives. Chem Heterocycl Comp 56, 1586–1591 (2020). https://doi.org/10.1007/s10593-020-02853-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02853-8