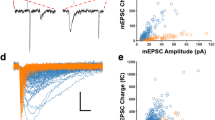
Abstract
Spontaneous activity in the brain maintains an internal structured pattern that reflects the external environment, which is essential for processing information and developing perception and cognition. An essential prerequisite of spontaneous activity for perception is the ability to reverberate external information, such as by potentiation. Yet its role in the processing of potentiation in mouse superior colliculus (SC) neurons is less studied. Here, we used electrophysiological recording, optogenetics, and drug infusion methods to investigate the mechanism of potentiation in SC neurons. We found that visual experience potentiated SC neurons several minutes later in different developmental stages, and the similarity between spontaneous and visually-evoked activity increased with age. Before eye-opening, activation of retinal ganglion cells that expressed ChR2 also induced the potentiation of spontaneous activity in the mouse SC. Potentiation was dependent on stimulus number and showed feature selectivity for direction and orientation. Optogenetic activation of parvalbumin neurons in the SC attenuated the potentiation induced by visual experience. Furthermore, potentiation in SC neurons was blocked by inhibiting the glutamate transporter GLT1. These results indicated that the potentiation induced by a visual stimulus might play a key role in shaping the internal representation of the environment, and serves as a carrier for short-term memory consolidation.







Similar content being viewed by others
References
Iemi L, Chaumon M, Crouzet SM, Busch NA. Spontaneous neural oscillations bias perception by modulating baseline excitability. J Neurosci 2017, 37: 807–819
Berkes P, Orban G, Lengyel M, Fiser J. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science 2011, 331: 83–87
Kenet T, Bibitchkov D, Tsodyks M, Grinvald A, Arieli A. Spontaneously emerging cortical representations of visual attributes. Nature 2003, 425: 954–956
Fu YX, Djupsund K, Gao H, Hayden B, Shen K, Dan Y. Temporal specificity in the cortical plasticity of visual space representation. Science 2002, 296: 1999–2003
Marques HG, Bharadwaj A, Iida F. From spontaneous motor activity to coordinated behaviour: a developmental model. PLoS Comput Biol 2014, 10: e1003653
Wang X-j. Probabilistic decision making by slow reverberation in cortical circuits. Neuron 2002, 36: 955–968
Mohajerani MH, Chan AW, Mohsenvand M, LeDue J, Liu R, McVea DA. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat Neurosci 2013, 16: 1426–1435
Luczak A, Bartho P, Harris KD. Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 2009, 62: 413–425
Han F, Caporale N, Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron 2008, 60: 321–327
Ackman JB, Crair MC. Role of emergent neural activity in visual map development. Curr Opin Neurobiol 2014, 24: 166–175
Chiu C, Weliky M. Spontaneous activity in developing ferret visual cortex in vivo. J Neurosci 2001, 15: 8906–8914
Bonetti C, Surace EM. Mouse embryonic retina delivers information controlling cortical neurogenesis. PLoS One 2010, 5: e15211
Hanson MG, Landmesser LT. Normal patterns of spontaneous activity are required for correct motor axon guidance and the expression of specific guidance molecules. Neuron 2004, 43: 687–701
Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science 1998, 279: 2108–2112
Kirkby LA, Sack GS, Firl A, Feller MB. A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 2013, 80: 1129–1144
Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci 2010, 11: 18–29
Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, et al. An instructive role for patterned spontaneous retinal activity in mouse visual map development. Neuron 2011, 70: 1115–1127
Wang K, Jiang T, Yu C, Tian L, Li J, Liu Y, et al. Spontaneous activity associated with primary visual cortex: a resting-state FMRI study. Cereb Cortex 2008, 18: 697–704
Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 1993, 361: 31–39
Bortolotto ZA, Fitzjohn SM, Collingridge GL. Roles of metabotropic glutamate receptors in LTP and LTD in the hippocampus. Curr Opin Neurobiol 1999, 9: 299–304
Katagiri H, Tanaka K, Manabe T. Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur J Neurosci 2001, 14: 547–553
Goubard V, Fino E, Venance L. Contribution of astrocytic glutamate and GABA uptake to corticostriatal information processing. J Physiol 2011, 589: 2301–2319
Tian SW, Yu XD, Cen L, Xiao ZY. Glutamate transporter GLT1 inhibitor dihydrokainic acid impairs novel object recognition memory performance in mice. Physiol Behav 2019, 199: 28–32
Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci 2007, 8: 935–947
Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L. Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr Opin Pharmacol 2015, 20: 116–123
He K, Huertas M, Hong SZ, Tie X, Hell JW, Shouval H, et al. Distinct eligibility traces for LTP and LTD in cortical synapses. Neuron 2015, 88: 528–538
Pita-Almenar JD, Collado MS, Colbert CM, Eskin A. Different mechanisms exist for the plasticity of glutamate reuptake during early long-term potentiation (LTP) and late LTP. J Neurosci 2006, 26: 10461–10471
Pita-Almenar JD, Zou S, Colbert CM, Eskin A. Relationship between increase in astrocytic GLT-1 glutamate transport and late-LTP. Learn Mem 2012, 19: 615–626
Tsvetkov E, Shin R-M, Bolshakov VY. Glutamate uptake determines pathway specificity of long-term potentiation in the neural circuitry of fear conditioning. Neuron 2004, 41: 139–151
Ito S, Feldheim DA. The Mouse superior colliculus: an emerging model for studying circuit formation and function. Front Neural Circuits 2018, 12: 10
Ellis EM, Gauvain G, Sivyer B, Murphy GJ. Shared and distinct retinal input to the mouse superior colliculus and dorsal lateral geniculate nucleus. J Neurophysiol 2016, 116: 602–610
Wang Q, Burkhalter A. Stream-related preferences of inputs to the superior colliculus from areas of dorsal and ventral streams of mouse visual cortex. J Neurosci 2013, 33: 1696–1705
Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, et al. A toolbox of cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 2012, 15: 793–802
Leng T, Gao X, Dilger JP, Lin J. Neuroprotective effect of lidocaine: is there clinical potential? Int J Physiol Pathophysiol Pharmacol 2016, 8: 9–13
Kalso E. Sodium channel blockers in neuropathic pain. Curr Pharm Des 2005, 11: 3005–3011
Seabrook TA, Burbridge TJ, Crair MC, Huberman AD. Architecture, function, and assembly of the mouse visual system. Annu Rev Neurosci 2017, 40: 499–538
Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci 2010, 30: 16573–16584
Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. J Neurosci 2008, 28: 7520–7536
Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature 2012, 490: 219–225
Wong RO. Retinal waves and visual system development. Annu Rev Neurosci 1999, 22: 29–47
Anand R. Chandrasekaran DTP, Ernesto Gonzalez,and Michael C. Crair. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci 2005, 25: 6929–6938.
Thyagarajan S, van Wyk M, Lehmann K, Lowel S, Feng G, Wassle H. Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J Neurosci 2010, 30: 8745–8758
Shi X, Barchini J, Ledesma HA, Koren D, Jin Y, Liu X, et al. Retinal origin of direction selectivity in the superior colliculus. Nat Neurosci 2017, 20: 550–558
Duan J, Fu H, Zhang J. Activation of parvalbumin-positive neurons in both retina and primary visual cortex improves the feature-selectivity of primary visual cortex neurons. Neurosci Bull 2017, 33: 255–263
Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A 1989, 86: 1698–1702
De Valois RL, Yund EW, Hepler N. The orientation and direction selectivity of cells in macaque visual cortex. Vision Res 1982, 22: 531–544
Kruskal JB, Wish M. Multidimensional scaling. SAGE Publications, London. 1978.
Maesschalck RD, Jouan-Rimbaud D, Massart DL. The mahalanobis distance. chemometrics & intelligent laboratory systems 2000, 50: 1–18.
Deza MM, Deza E. Encyclopedia of distances. Reference Reviews 2009, 24: 1–583
Lundstrom BN, Fairhall AL. Decoding stimulus variance from a distributional neural code of interspike intervals. J Neurosci 2006, 26: 9030–9037
Gong LQ, He LJ, Dong ZY, Lu XH, Poo MM, Zhang XH. Postinduction requirement of NMDA receptor activation for late-phase long-term potentiation of developing retinotectal synapses in vivo. J Neurosci 2011, 31: 3328–3335
Xu S, Jiang W, Poo MM, Dan Y. Activity recall in a visual cortical ensemble. Nat Neurosci 2012, 15(449–455): S441-442
Lynch MA. Long-term potentiation and memory. Physiol Rev 2004, 84: 87–136
Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci 2010, 30: 16304–16313
Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci 2008, 31: 479–509
Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron 2012, 75: 230–249
Li N, Gu Y. The visual pathway for binocular integration. Neurosci Bull 2020, 36: 1089–1091.
White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature 2001, 411: 1049–1052
Hensch TK. Critical period regulation. Annu Rev Neurosci 2004, 27: 549–579
McLaughlin T, Torborg CL, Feller MB, O’Leary DDM. Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 2003, 40: 1147–1160
Hensch MFTK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 2000, 404: 4
Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 1998, 282: 1504–1508
Harauzov A, Spolidoro M, DiCristo G, De Pasquale R, Cancedda L, Pizzorusso T, et al. Reducing intracortical inhibition in the adult visual cortex promotes ocular dominance plasticity. J Neurosci 2010, 30: 361–371
Maya-Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 2008, 320: 385–388
Sale A, Maya-Vetencourt JF, Medinin P, Cenni MC, Baroncelli L, De Pasquale R, et al. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci 2007, 10: 679–681
Gao WJ, Wormington AB, Newman DE, Pallas SL. Development of inhibitory circuitry in visual and auditory cortex of postnatal ferrets: immunocytochemical localization of calbindin- and parvalbumin-containing neurons. J Comp Neurol 2000, 422: 140–157
Krishnan K, Wang B-S, Lu J, Wang L, Maffei A, Cang J, et al. MeCP2 regulates the timing of critical period plasticity that shapes functional connectivity in primary visual cortex. Proc Natl Acad Sci U S A 2015, 112: E4782–E4791
Shi J, Li Q, Wen T. Dendritic cell factor 1-knockout results in visual deficit through the GABA system in mouse primary visual cortex. Neurosci Bull 2018, 34: 465–475
Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron 2013, 77: 388–405
Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 2011, 34: 205–231
Alloway KD, Smith JB, Beauchemin KJ. Quantitative analysis of the bilateral brainstem projections from the whisker and forepaw regions in rat primary motor cortex. J Comp Neurol 2010, 518: 4546–4566
Killackey HP, RS. E. Trigeminal projections of the superior Colliculus of the rat. J Comp Neurol 1981, 201: 221–242.
Sparks DL, Lee C, Rohrer WH. Population coding of the direction, amplitude, and velocity of saccadic eye movements by neurons in the superior colliculus. Cold Spring Harb Symp Quant Biol 1990, 55: 805–811
Liang F, Xiong XR, Zingg B, Ji XY, Zhang LI, Tao HW. Sensory cortical control of a visually induced arrest behavior via corticotectal projections. Neuron 2015, 86: 755–767
Shang C, Liu Z, Chen Z, Shi Y, Wang Q, Liu S, et al. A parvalbumin-positive excitatory visual pathway to trigger fear responses in mice. Science 2015, 348: 1472–1477.
Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain 2006, 129: 1659–1673
Adams JP, Dudek SM. Late-phase long-term potentiation: getting to the nucleus. Nat Rev Neurosci 2005, 6: 737–743
Yukio Seki PJF, Richard W. Keller, Bruce I. Tranmer, Harold K. Kimelberg. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke 1999, 30: 433–440.
Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA Jr, Ongur D, Cohen BM. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 2010, 35: 2049–2059
May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 2006, 151: 321–378
Feinberg EH, Meister M. Orientation columns in the mouse superior colliculus. Nature 2015, 519: 229–232
Kerschensteiner D. Glutamatergic retinal waves. Front Neural Circuits 2016, 10: 38
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771195, 81790640 and 82021002), a Shanghai Municipal Science and Technology Major Project (2018SHZDZX01) and ZJLab, Key Scientific Technological Innovation Research Project of the Ministry of Education, Sanming Project of Medicine in Shenzhen (SZSM202011015) and Shanghai Health and Family Planning Commission (20164Y0096, 20184Y0184).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors claim that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Q., Fu, H., Wang, G. et al. Short-Term Visual Experience Leads to Potentiation of Spontaneous Activity in Mouse Superior Colliculus. Neurosci. Bull. 37, 353–368 (2021). https://doi.org/10.1007/s12264-020-00622-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00622-3