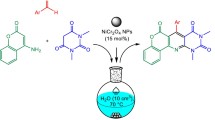
Abstract
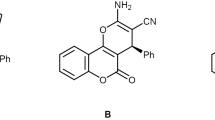
An improved one-pot three-component reaction of carbonyl compounds (dimedon, 4-hydroxy coumarin and 1,3-cyclohexadion) with malononitrile and aryl aldehydes in aqueous media (H2O:EtOH) as a solvent with short reaction time for the synthesis of benzo[b]pyran and 3,4- dihydropyrano[c]chromane derivatives by using nanostructured coumarin-based cobalt complex as an efficient heterogeneous catalyst. The salient features of this new protocol include simple procedure, high yields, easy isolation of products without need to column chromatography and short reaction times. Also, the nanocatalyst was recovered by adding EtOH and reused five times without significant loss of its catalytic activity. Biological impact assessments of the complex were conducted by DNA cleavage ability assay and eukaryotic cell toxicity measurement. Although the complex showed single-strand DNA breakage under oxidative conditions, its high polar nature revealed a marked decrease in cell toxicity data due to the restriction on cell entry.
Graphic abstract








Similar content being viewed by others
References
U.R. Pratap, D.V. Jawale, P.D. Netankar, R.A. Mane, Tetrahedron Lett. 52, 5817 (2011)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
W.O. Foye, Prinicipi di Chemica Farmaceutica (Piccin, Padova, 1991), p. 416
M. Darbarwar, V. Sundaramurthy, Synthesis 1982, 337 (1982)
A. Shaabani, M.M. Amini, S. Ghasemi, R. Ghadari, A.H. Rezayan, Y. Fazaeli, S. Feizi, Chem. Pharm. Bull. 58, 270 (2010)
T.S. Jin, R.Q. Zhao, T.S. Li, Arkivoc 11, 176 (2006)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
C.S. Konkoy, D.B. Fick, S.X. Cai, N.C. Lan, J.F.W. Keana, PCT Int Appl WO 0075123, 2000 In Chem. Abstr. 134, 29313a (2001)
L.M. Wang, J.H. Shao, H. Tian, Y.H. Wang, B. Liu, J. Fluor. Chem. 127, 97 (2006)
X.Z. Lian, Y. Huang, Y.Q. Li, W.J. Zheng, Monatsh. Chem. 139, 129 (2008)
M. Beerappa, K. Shivashankar, RSC Adv. 5, 30364 (2015)
O. Rosati, A. Pelosi, A. Temperini, V. Pace, M. Curini, Synthesis 48, 1533 (2016)
P. Rai, A. Ibad, H. Sagir, I.R. Siddiqui, ChemistrySelect 1, 1300 (2016)
N. Azizi, T.S. Ahooie, M.M. Hashemi, I. Yavari, Synlett 29, 645 (2018)
S. Gao, C.H. Tsai, C. Tseng, C.F. Yao, Tetrahedron 64, 9143 (2008)
A. Boumoud, A. Yahiaoui, T. Boumoud, A. Debache, J. Chem. Pharm. Res. 4, 795 (2012)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
D.M. Pore, K.A. Undale, B.B. Dongare, U.V. Desai, Catal. letters 132, 104 (2009)
D.Q. Shi, S. Zhang, Q.Y. Zhuang, S.J. Tu, H.W. Hu, Chinese. J. Org. Chem. 23, 877 (2003)
R. Hekmatshoar, S. Majedi, K. Bakhtiari, Catal. Commun. 9, 307 (2008)
S. Gurumurthi, V. Sundari, R. Valliappan, J. Chem. 6, S466 (2009)
J.M. Khurana, S. Kumar, Tetrahedron Lett. 50, 4125 (2009)
D. Kumar, V.B. Reddy, B.G. Mishra, R.K. Rana, M.N. Nadagouda, R.S. Varma, Tetrahedron 63, 3093 (2007)
A. Khalafi-Nezhad, M. Nourisefat, F. Panahi, Org. Biomol. Chem. 13, 7772 (2015)
Y.M. Litvinov, V.Y. Mortikov, A.M. Shestopalov, J. Comb. Chem. 10, 741 (2008)
H.R. Safaei, M. Shekouhy, A. Shirinfeshan, S. Rahmanpur, Mol. Divers. 16, 669 (2012)
H. Azizi, A. Khorshidi, K. Tabatabaeian, J. Iran. Chem. Soc. 15, 1023 (2018)
A. Mulik, P. Hegade, S. Mulik, M. Deshmukh, Res. Chem. Intermed. 45, 5641 (2019)
M.P. Lati, F. Shirini, M. Alinia-Asli, M.A. Rezvani, J. Nanosci. Nanotechnol. 20, 973 (2020)
H. Sharghi, M. Jokar, Heterocycles 71, 2721 (2007)
K.B. Gudasi, M.S. Patil, R.S. Vadavi, Eur. J. Med. Chem. 43, 2436 (2008)
H. Sharghi, S.F. Razavi, M. Aberi, F. Tavakoli, M. Shekouhy, ChemistrySelect 5, 2662 (2020)
M.G. Dekamin, M. Eslami, Green Chem. 16, 4914 (2014)
M. Abdollahi-Alibeik, F. Nezampour, React. Kinet. Mech. Catal. 108, 213 (2013)
H.R. Saadati-Moshtaghin, F.M. Zonoz, Mater. Chem. Phys. 199, 159 (2017)
J.M. Khurana, B. Nand, P. Saluja, Tetrahedron 66, 5637 (2010)
M.A. Nasseri, S.M. Sadeghzadeh, J. Iran. Chem. Soc. 10, 1047 (2013)
R. Nongrum, G.S. Nongthombam, M. Kharkongor, J. Rani, N. Rahman, C. Kathing, B. Myboh, R. Nongkhlaw, RSC Adv. 6, 108384 (2016)
A. Gupte, R.J. Mumper, Cancer Treat. Rev. 35, 32 (2009)
F. Denizot, R. Lang, J. Immunol. Methods 89, 271 (1986)
J. Balou, M.A. Khalilzadeh, D. Zareyee, Sci. Rep. 9, 1 (2019)
M.S. Mirhosseyni, F. Nemati, A. Elhampour, Comb. Chem. High. T Scr. 21, 87 (2018)
A. Maleki, Z. Hajizadeh, Silicon 11, 2789 (2019)
M. Khoobi, L. Mamani, F. Rezazadeh, Z. Zareie, A. Foroumadi, A. Ramazani, A. Shafiee, J. Mol. Catal. A: Chem. 359, 74 (2012)
H. R. Saadati-Moshtaghin, F. Abbasinohoji, Polycyclic. Aromat. Compd. 1 (2019)
M. Salimi, M.A. Nasseri, B.N. Jazi, J. Iran. Chem. Soc. 16, 2221 (2019)
N.N. Pesyan, G.R. Bardajee, E. Kashani, M. Mohammadi, H. Batmani, Res. Chem. Intermed. 46, 347 (2020)
A. Shaabani, S. Samadi, Z. Badri, A. Rahmati, Catal. Letters 104, 39 (2005)
S. Balalaie, M. Sheikh-Ahmadi, M. Bararjanian, Catal. Commun. 8, 1724 (2007)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 7847 (2015)
T.B. Kryston, A.B. Georgiev, P. Pissis, A.G. Georgakilas, Mutat. Res-Fund. Mol. M. 711, 193 (2011)
L.F. Stadlmair, S. Grosse, T. Letzel, J.E. Drewes, J. Grassmann, Anal. Bioanal. Chem. 411, 339 (2019)
J.B. Wang, D.S. Cao, M.F. Zhu, Y.H. Yun, N. Xiao, Y.Z. Liang, J. Chemom. 29, 389 (2015)
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharghi, H., Razavi, S.F., Aberi, M. et al. Nanostructured coumarin-based cobalt complex as an efficient, heterogeneous and recyclable catalyst for the three-component synthesis of benzo[b]pyran and 3,4-dihydropyrano[c]chromene derivatives. J IRAN CHEM SOC 18, 1641–1655 (2021). https://doi.org/10.1007/s13738-020-02136-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02136-1