Abstract
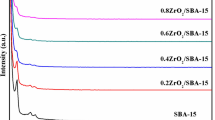
Mesoporous MnO2/SBA-15 was prepared by a facile route using KMnO4 and MnCl2 as Mn source and used for removal of Cs+. The effects of MnO2 content, initial Cs+ concentration, contact time and pH on the Cs+ adsorption behavior were investigated. It was found that Cs+ adsorption capacity of SBA-15 can be significantly improved by MnO2 loading. The adsorption capacity increased with increasing MnO2 content. Furthermore, the adsorption data can be well fitted by the Langmuir model, and the calculated maximum adsorption capacity of 80%MnO2/SBA-15 is 104.8 mg/g. The Cs+ adsorption process can be better described by the pseudo-second-order model.










Similar content being viewed by others
References
Mathur JN, Murali MS, Nash KL (2001) Actinide partitioning: a review. Solv Extr Ion Exch 19:357–390
Deng H, Li YX, Wu L (2016) The novel composite mechanism of ammonium molybdophosphate loaded on silica matrix and its ion exchange breakthrough curves for cesium. J Hazard Mater 324:348–356
Rogers H, Bowers J, Gates-Anderson D (2012) An isotope dilution-precipitation process for removing radioactive cesium from wastewater. J Hazard Mater 243:124–129
Park Y, Shin WS, Choi SJ (2013) Ammonium salt of heteropoly acid immobilized on mesoporous silica (SBA-15): an efficient ion exchanger for cesium ion. Chem Eng J 220:204–213
Schneider S, Garcez AC, Mélodie T, François B, Larivière D, Kleitz F (2013) Nanoporous ammonium molybdophosphate-silica hybrids as regenerable ultra-selective extraction agents for radiocesium monitoring. New J Chem 37:3877–3880
Dietz ML, Philip EH, Rhoads S, Bartsch RA, Krzykawski J (1996) Extraction of cesium from acidic nitrate media using macrocyclic polyethers: the role of organic phase water. Solv Extr Ion Exch 14:1–12
Mahendra C, Bera S, Babu CA, Rajan KK (2013) Separation of cesium by electro dialysis ion exchange using AMP-PAN. Sep Purif Technol 48:2473–2478
Ye XS, Wu ZJ, Li W, Liu HN, Qing BJ, Guo M, Ge F (2009) Rubidium and cesium ion adsorption by an ammonium molybdophosphate-calcium alginate composite adsorbent. Colloid Surface A 342:76–83
Torad NL, Hu M, Imura M, Naito M, Yamauchi Y (2012) Large Cs adsorption capability of nanostructured prussian blue particles with high accessible surface areas. J Mater Chem 22:18261–18267
Avramenko V, Bratskaya S, Zheleznov V, Sheveleva I, Voitenko O, Sergienko V (2011) Colloid stable sorbents for cesium removal: preparation and application of latex particles functionalized with transition metals ferrocyanides. J Hazard Mater 186:1343–1350
Dan H, Xian Q, Chen L, Xiong TH, Jiang ZD, Yi FC, Ding Y (2019) One-step direct synthesis of mesoporous AMP/SBA-15 using PMA as acid media and its use in cesium ion removal. J Nucl Mater 527:151809
Wang XG, Lin KSK, Chan JCC, Cheng SF (2005) Direct synthesis and catalytic applications of ordered large pore aminopropyl-functionalized SBA-15 mesoporous materials. J Phys Chem B 109:1763–1769
Gao DW, Duan AJ, Zhang X, Zhao Z, Hong E, Li JM, Wang H (2015) Synthesis of NiMo catalysts supported on mesoporous Al-SBA-15 with different morphologies and their catalytic performance of DBT HDS. Appl Catal B Environ 165:269–284
Katiyar A, Yadav S, Smirniotis PG, Pinto NG (2006) Synthesis of ordered large pore SBA-15 spherical particles for adsorption of biomolecules. J Chromatogr A 1122:13–20
Cao L, Kruk M (2011) Facile method to synthesize platelet SBA-15 silica with highly ordered large mesopores. J Colloid Interf Sci 361:472–476
Dan H, Chen LY, Xian Q, Yi FC, Ding Y (2019) Tailored synthesis of SBA-15 rods using different types of acids and its application in adsorption of uranium. Sep Purif Technol 210:491–496
Aghayan H, Khanchi AR, Yousefi T, Ghasemi H (2017) Tungsten substituted molybdophosphoric acid loaded on various types of mesoporous silica SBA-15 for application of thorium ion adsorption. J Nucl Mater 496:207–214
Dan H, Xian Q, Chen LY, Chen L, Wu SF, Yi FC, Ding Y (2019) Fabrication of AMP/SBA-15 with various morphologies for cesium removal from aqueous solution. J Sol Gel Sci Technol 91:165–177
Yuan LY, Liu YL, Shi WQ, Li ZJ, Lan JH, Feng YX, Zhao YL, Yuan YL, Chai ZF (2012) A novel mesoporous material for uranium extraction, dihydroimidazole functionalized SBA-15. J Mater Chem 22:17019–17026
Ji GJ, Zhu GR, Wang XH, Wei YL, Yuan JS, Gao CJ (2017) Preparation of amidoxime functionalized SBA-15 with platelet shape and adsorption property of U(VI). Sep Purif Technol 174:455–465
Zhi B, Ding H, Wang DM, Cao Y, Zhang Y, Wang X, Liu YL, Huo QS (2014) Ordered mesoporous MnO2 as a synergetic adsorbent for effective arsenic(III) removal. J Mater Chem A 2:2374–2382
Valsala TP, Joseph A, Sonar NL, Sonavane MS, Shah JG, Raj K, Venugopal V (2010) Separation of strontium from low level radioactive waste solutions using hydrous manganese dioxide composite materials. J Nucl Mater 404:138–143
Pan N, Li L, Ding J, Li SK, Wang RB, Jin YD, Wang XK, Xia CQ (2016) Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J Hazard Mater 309:107–115
Kanungo SB, Tripathy SS, Mishra SK, Rajeev BS (2004) Adsorption of Co2+, Ni2+, Cu2+, and Zn2+ onto amorphous hydrous manganese dioxide from simple (1-1) electrolyte solutions. J Colloid Interf Sci 269:11–21
Hasany SM, Chaudhary MH (1981) Adsorption studies of strontium on manganese dioxide from aqueous solutions. Int J Ap Mat Com-Pol 32:899–904
White DA, Labayru R (1991) Synthesis of a manganese dioxide-silica hydrous composite and its properties as a sorbtion material for strontium. Ind Eng Chem Res 30:207–210
Gheju M, Balcu I, Mosoarca G (2016) Removal of Cr(VI) from aqueous solutions by adsorption on MnO2. J Hazard Mater 310:270–277
Ivanets AI, Prozorovich GV, Kouznetsova TF, Radkevich AV, Zarubo AM (2016) Mesoporous manganese oxides prepared by sol-gel method: synthesis, characterization and sorption properties towards strontium ions. Environ Nanotechnol Monit Manag 6:261–269
Ivanets AI, Katsoshvili LL, Krivoshapkin PV, Pozorovich VG, Kuznetsova TF, Krivoshapkina EF, Radlkevich AV, Zarubo AM (2017) Sorption of strontium ions onto mesoporous manganese oxide of OMS-2 type. Radiochemistry 59:264–271
Ivanets AI, Milyutin VV, Prozorovich VG, Kuznetsova TF, Petrovskaya AO, Nekrasova NA (2019) Sorption of 90Sr onto manganese oxides prepared in aqueous-ethanol media. Radiochemistry 61:707–713
Pendelyuk OI, Lisnycha TV, Strelko VV, Kirillov SA (2005) Amorphous MnO2-TiO2 composites as sorbents for Sr2+ and UO22+. Adsorption 11:799–804
Luo C, Wei RY, Guo D, Zhang SF, Yan SQ (2013) Adsorption behavior of MnO2 functionalized multi-walled carbon nanotubes for the removal of cadmium from aqueous solutions. Chem Eng J 225:406–415
Krivoshapkin PV, Ivanets AI, Torlopov MA, Mikhaylov VI, Srivastava V, Sillanpää M, Prozorovich VG, Kouznetsova TF, Koshevaya ED, Krivoshapkina EF (2019) Nanochitin/manganese oxide-biodegradable hybrid sorbent for heavy metal ions. Carbohyd Polym 210:135–143
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure App Chem. https://doi.org/10.1515/pac-2014-1117
Ding Y, Dan H, Lu XR, Wu YL, Yuan SB, Mao XL (2014) Facile route to synthesize mesoporous silica SBA-15 platelets. Mater Chem Phys 148:17–20
Liu X, Wang J (2020) Electro-assisted adsorption of Cs(I) and Co(II) from aqueous solution by capacitive deionization with activated carbon cloth/graphene oxide composite electrode. Sci Total Environ 749:141524
Liu X, Wu J, Hou L, Wang J (2019) Removal of Co, Sr and Cs ions from simulated radioactive wastewater by forward osmosis. Chemosphere 232:87–95
Nightingale ERJ (1959) Phenomenological theory of ion solvation. Effective radii of hydrated ions. BBA Mol Basis Dis 63:566–567
Hong HJ, Kim BG, Hong J, Ryu J, Ryu T, Chung KS, Kim H, Park IS (2017) Enhanced Sr adsorption performance of MnO2-alginate beads in seawater and evaluation of its mechanism. Chem Eng J 319:163–169
Tan LC, Wang J, Liu Q, Sun YB, Jing XY, Liu LH, Liu JY, Song DL (2015) The synthesis of a manganese dioxide-iron oxide-graphene magnetic nanocomposite for enhanced uranium(VI) removal. New J Chem 39:868–876
Wang N, Chu W, Zhang T, Zhao XS (2012) Synthesis, characterization and catalytic performances of Ce-SBA-15 supported nickel catalysts for methane dry reforming to hydrogen and syngas. Int J Hydrog Energ 37:19–30
Yang BW, Gong QJ, Zhao LP, Sun H, Ren NN, Qin JX, Xu J, Yang HY (2011) Preconcentration and determination of lead and cadmium in water samples with a MnO2 coated carbon nanotubes by using ETAAS. Desalination 278:65–69
Dan H, Ding Y, Lu XR, Chi FT, Yuan SB (2016) Adsorption of uranium from aqueous solution by mesoporous SBA-15 with various morphologies. J Radioanal Nucl Chem 310:1–8
Tran HN, You SJ, Hosseini-Bandegharaei Chao AHP (2017) Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: a critical review. Water Res 120:88–116
Ho S (2006) Review of second-order models for adsorption systems. J Hazard Mater 136:681–689
Ghaemi A, Torab-Mostaedi M, Ghannadi-Maragheh M (2011) Characterizations of strontium(II) and barium(II) adsorption from aqueous solutions using dolomite powder. J Hazard Mater 190:916–921
Willms C, Li Z, Allen L, Evans CV (2004) Desorption of cesium from kaolinite and illite using alkylammonium salts. Appl Clay Sci 25:125–133
Adebowale KO, Unuabonah IE, Olu-Owolabi BI (2006) The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J Hazard Mater 134:130–139
Maslova M, Mudruk N, Ivanets AI, Shashkova I, Kitikova N (2019) A novel sorbent based on Ti-Ca-Mg phosphates: synthesis, characterization, and sorption properties. Environ Sci Pollut Res 27:1–17
Ivanets A, Kitikova N, Shashkova I, Radkevich A, Stepanchuk T, Maslova M, Mudruk N (2020) One-stage adsorption treatment of liquid radioactive wastes with complex radionuclide composition. Water Air Soil Pollut 231:144
Ivanets AI, Shashkova II, Drozdova NV, Davydov DY, Radkevich AV (2014) Recovery of cesium ions from aqueous solutions with composite sorbents based on tripolite and copper(II) and nickel(II) ferrocyanides. Radiochemistry 56:524–528
Chakravarty R, Ram R, Pillai KT, Pamale Y, Kamat RV, Dash A (2012) Ammonium molybdophosphate impregnated alumina microspheres as a new generation sorbent for chromatographic 137Cs/137mBa generator. J Chromatogr A 1220:82–91
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22006123), the National Defense Basic Scientific Research Program (No. JCKY2019404D001), the application basic research project of Science and Technology Department of Sichuan Province (No. 20YYJC3547), the Undergraduate Targeted Aid Innovation Fund of Southwest University of Science and Technology (No. JZ20-041, JZ20-045), and the Undergraduate Innovation Fund of Southwest University of Science and Technology (CX20-049, CX20-053).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xian, Q., He, X., Wang, E. et al. Preparation of mesoporous MnO2/SBA-15 and its cesium ion adsorption properties. J Radioanal Nucl Chem 327, 505–512 (2021). https://doi.org/10.1007/s10967-020-07522-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07522-w