Abstract
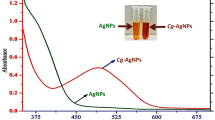
The measurement of total antioxidant capacity (TAC) indicating the cooperative action of all antioxidants in a complex sample is an important analytical challenge. A novel green silver nanoparticle-based antioxidant capacity (GSNP-AC) method using carob extract was designed for TAC measurement. Green synthesis of nanoparticles has various advantages such as environmentally friendliness, reliability, sustainability and fast production. In the presence of silver seeds formed with carob extract, Ag+ is reduced to spherical silver nanoparticles (SNPs) with antioxidants, resulting in enlarged particles with a symmetric surface plasmon resonance (SPR) absorption band at 434 nm. The SPR of SNPs allowed quantification of antioxidants (i.e., increase in SPR absorbance being correlated to antioxidant concentration), and the GSNP-AC method gave a linear response over a wide concentration range of antioxidants. The molar absorptivity and LOD for trolox were 11700 L mol−1 cm−1 and 0.31 µM, respectively. The proposed method was validated in terms of concentration-dependent linear response, additivity of absorbances, precision and accuracy. The results obtained with the proposed method for standard antioxidants, synthetic mixtures and real sample extracts were correlated with those of the traditional CUPRAC method. This green synthesized silver nanoparticle-based TAC method showed simple, rapid, cost-effective, and eco-friendly superiorities over similar other nanoparticle-based assays.
Graphic Abstract











Similar content being viewed by others
References
Gupta, R.K., Patel, A.K., Shah, N., Choudhary, A.K., Jha, U.K., Yadav, U.C., Gupta, P.K.., Pakuwal, U: Oxidative stress and antioxidants in disease and cancer: a review. Asian Pac. J. Cancer. P. 15, 4405–4409 (2014)
Ozougwu, J.C., Obiukwu, C.E., Obimba, K.C., Elom, M.O., Usanga, V.U.: Haematological changes associated with male and female typhoid fever patients. Int. J. Res. Pharm. Biosci. 3, 21–26 (2016)
Apak, R., Çapanoğlu, E., Arda, A.Ü.: Nanotechnological Methods of Antioxidant Characterization. In: Guthrie, B., Beauchamp, J., Buettner, A., Lavine, B.K. (eds.) The Chemical Sensory Informatics of Food: Measurement, pp. 209–234. Analysis, Integration, American Chemical Society, Washington DC (2015)
Singh, P., Kim, Y.J., Zhang, D., Yang, D.C.: Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 34, 588–599 (2016)
Ahmad, Z., Afreen, A., Mehmood, M., Ali, I., Asgher, R., Aziz, M.: One-step synthesis of Ag nano-assemblies and study of their antimicrobial activities. J. Nanostructure Chem. 5, 325–331 (2015)
Zhang, X.F., Liu, Z.G., Shen, W., Gurunathan, S.: Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 17, 1534 (2016)
Rolim, W.R., Pelegrino, M.T., de Araújo Lima, B., Ferraz, L.S., Costa, F.N., Bernardes, J.S., Rodigues, T., Brocchi, A.B., Seabra, A.B.: Green tea extract mediated biogenic synthesis of silver nanoparticles: characterization, cytotoxicity evaluation and antibacterial activity. Appl. Surf. Sci. 463, 66–74 (2019)
Singh, J., Dutta, T., Kim, K.H., Rawat, M., Samddar, P., Kumar, P.: ‘Green’synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnol. 16, 84 (2018)
Khandel, P., Yadaw, R.K., Soni, D.K., Kanwar, L., Shahi, S.K.: Biogenesis of metal nanoparticles and their pharmacological applications: present status and application prospects. J. Nanostructure Chem. 8, 217–254 (2018)
Pirtarighat, S., Ghannadnia, M., Baghshahi, S.: Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 9, 1–9 (2019)
Maity, D., Pattanayak, S., Mollick, M.M.R., Rana, D., Mondal, D., Bhowmick, B., Das, S.K., Chattopadhyay, S., Das, B., Roy, S., Chakraborty, M., Chattopadhyay, D.: Green one step morphosynthesis of silver nanoparticles and their antibacterial and anticancerous activities. New J. Chem. 40, 2749–2762 (2016)
Mollick, M. M. R., Bhowmick, B., Mondal, D., Maity, D., Rana, D., Dash, S. K., Chattopadhyay, S., Roy, S., Sarkar, J., Acharya, K., Chakraborty, M., Chattopadhyay, D.: Anticancer (in vitro) and antimicrobial effect of gold nanoparticles synthesized using Abelmoschus esculentus (L.) pulp extract via a green route. RSC Adv. 4, 37838–37848 (2014).
Mollick, M.M.R., Bhowmick, B., Maity, D., Mondal, D., Roy, I., Sarkar, J., Rana, D., Acharya, K., Chattopadhyay, S., Chattopadhyay, D.: Green synthesis of silver nanoparticles-based nanofluids and investigation of their antimicrobial activities. Microfluid. Nanofluid. 16, 541–551 (2014)
Stavrou, I.J., Christou, A., Kapnissi-Christodoulou, C.P.: Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 269, 355–374 (2018)
Dhaouadi, K., Belkhir, M., Akinocho, I., Raboudi, F., Pamies, D., Barrajón, E., Estevan, S., Fattouch, S.: Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT-Food Sci. Technol. 57, 1–8 (2014)
Zhu, B.J., Zayed, M.Z., Zhu, H.X., Zhao, J., Li, S.P.: Functional polysaccharides of carob fruit: a review. Chin. Med. 14, 40 (2019)
Mantri, Y., Davidi, B., Lemaster, J.E., Hariri, A., Jokerst, J.V.: Iodide-doped precious metal nanoparticles: measuring oxidative stress in vivo via photoacoustic imaging. Nanoscale 12, 10511–10520 (2020)
Apak, R.: Current issues in antioxidant measurement. J. Agr. Food Chem. 67, 9187–9202 (2019)
Özyürek, M., Güngör, N., Baki, S., Güçlü, K., Apak, R.: Development of a silver nanoparticle-based method for the antioxidant capacity measurement of polyphenols. Anal. Chem. 84, 8052–8059 (2012)
Xu, W., Zhang, F., Luo, Y., Ma, L., Kou, X., Huang, K.: Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohyd. Res. 344, 217–222 (2009)
Huang, H., Yang, X.: Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohyd. Res. 339, 2627–2631 (2004)
Marambio-Jones, C., Hoek, E.M.: A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 12, 1531–1551 (2010)
Apak, R., Güçlü, K., Özyürek, M., Karademir, S.E.: Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agr. Food Chem. 52, 7970–7981 (2004)
Miller, J.C., Miller, J.N.: Errors in instrumental analysis; regression and correlation. In: Horwood E, Prentice H (eds) Statistics for analytical chemistry, 3rd edn. New York and London, pp. 101–139 (1993)
Scampicchio, M., Wang, J., Blasco, A.J., Sanchez Arribas, A., Mannino, S., Escarpa, A.: Nanoparticle-based assays of antioxidant activity. Anal. Chem. 78, 2060–2063 (2006)
Szydłowska-Czerniak, A., Tułodziecka, A., Szłyk, E.: A silver nanoparticle-based method for determination of antioxidant capacity of rapeseed and its products. Analyst 137, 3750–3759 (2012)
Tułodziecka, A., Szydłowska-Czerniak, A.: Development of a novel gold nanoparticle-based method to determine antioxidant capacity of Brassica oilseeds, white flakes and meal. Food Chem. 208, 142–149 (2016)
Ahmad, N., Sharma, S., Alam, M.K., Singh, V.N., Shamsi, S.F., Mehta, B.R., Fatma, A.: Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloid. Surface. B 81, 81–86 (2010)
Jiang, Z.J., Liu, C.Y.: Seed-mediated growth technique for the preparation of a silver nanoshell on a silica sphere. J. Phys. Chem. B 107, 12411–12415 (2003)
Farag, M.A., El-Kersh, D.M.: Volatiles profiling in Ceratonia siliqua (Carob bean) from Egypt and in response to roasting as analyzed via solid-phase microextraction coupled to chemometrics. J. Adv. Res. 8, 379–385 (2017)
Bhattarai, B., Zaker, Y., Bigioni, T.P.: Green synthesis of gold and silver nanoparticles: Challenges and opportunities. Curr. Opin. Green Sustain. Chem. 12, 91–100 (2018)
Raveendran, P., Fu, J., Wallen, S.L.: Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 125, 13940–13941 (2003)
Elia, P., Zach, R., Hazan, S., Kolusheva, S., Porat, Z.E., Zeiri, Y.: Green synthesis of gold nanoparticles using plant extracts as reducing agents. Int. J. Nanomed. 9, 4007–4021 (2014)
Balavandy, S.K., Shameli, K., Biak, D.R.B.A., Abidin, Z.Z.: Stirring time effect of silver nanoparticles prepared in glutathione mediated by green method. Chem. Cent. J. 8, 11 (2014)
Coseri, S., Spatareanu, A., Sacarescu, L., Rimbu, C., Suteu, D., Spirk, S., Harabagiu, V.: Green synthesis of the silver nanoparticles mediated by pullulan and 6-carboxypullulan. Carbohyd. Polym. 116, 9–17 (2015)
Çelik, S.E., Bekdeşer, B., Apak, R.: A novel colorimetric sensor for measuring hydroperoxide content and peroxyl radical scavenging activity using starch-stabilized gold nanoparticles. Talanta 196, 32–38 (2019)
Gurunathan, S., Han, J., Park, J.H., Kim, J.H.: A green chemistry approach for synthesizing biocompatible gold nanoparticles. Nanoscale Res. Lett. 9, 1–11 (2014)
Singh, P., Kim, Y.J., Yang, D.C.: A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif. Cell Nanomed. B 44, 1949–1957 (2016)
Okafor, F., Janen, A., Kukhtareva, T., Edwards, V., Curley, M.: Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int. J. Env. Res. Pub. He. 10, 5221–5238 (2013)
Siegel, J., Kvítek, O., Ulbrich, P., Kolská, Z., Slepička, P., Švorčík, V.: Progressive approach for metal nanoparticle synthesis. Mater. Lett. 89, 47–50 (2012)
Moores, A., Goettmann, F.: The plasmon band in noble metal nanoparticles: an introduction to theory and applications. New. J. Chem. 30, 1121–1132 (2006)
Goulas, V., Stylos, E., Chatziathanasiadou, M.V., Mavromoustakos, T., Tzakos, A.G.: Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 17, 1875 (2016)
Quiles-Carrillo, L., Mellinas, C., Garrigós, M.D.C., Balart, R., Torres-Giner, S.: Optimization of microwave-assisted extraction of phenolic compounds with antioxidant activity from carob pods. Food Anal. Methods 12, 2480–2490 (2019)
Dinesh, V.P., Biji, P., Ashok, A., Dhara, S.K., Kamruddin, M., Tyagi, A.K., Raj, B.: Plasmon-mediated, highly enhanced photocatalytic degradation of industrial textile dyes using hybrid ZnO@Ag core-shell nanorods. RSC Adv. 4, 58930–58940 (2014)
Ledet, G., Bostanian, L.A., Mandal, T.K.: Nanoemulsions as a vaccine adjuvant. In: Tiwari, A. Tiwari (eds.) Bioengineered Nanomaterials, CRC Press: Boca Raton, pp. 125–148 (2013)
Rice-Evans, C.A., Miller, N.J., Paganga, G.: Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Bio. Med. 20, 933–956 (1996)
Robards, K., Prenzler, P.D., Tucker, G., Swatsitang, P., Glover, W.: Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 66, 401–436 (1999)
Rice-Evans, C., Miller, N., Paganga, G.: Antioxidant properties of phenolic compounds. Trends Plant Sci. 2, 152–159 (1997)
Apak, R., Güçlü, K., Özyürek, M., Çelik, S.E.: Mechanism of antioxidant capacity assays and the CUPRAC (cupric ion reducing antioxidant capacity) assay. Microchim. Acta 160, 413–419 (2008)
Taguchi, R., Hatayama, K., Takahashi, T., Hayashi, T., Sato, Y., Sato, D., Ohta, K., Nakano, H., Seki, C., Ende, Y., Tokuraku, K., Uwai, K.: Structure–activity relations of rosmarinic acid derivatives for the amyloid β aggregation inhibition and antioxidant properties. Eur. J. Med. Chem. 138, 1066–1075 (2017)
Apak, R., Güçlü, K., Demirata, B., Özyürek, M., Çelik, S.E., Bektaşoğlu, B., Berker, K.I., Özyurt, D.: Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 12, 1496–1547 (2007)
Acknowledgements
The authors thank Prof. Dr. İsmail Tuncer Değim for sharing his research infrastructures. The authors also thank Istanbul University-Cerrahpasa, Inorganic Chemistry Department for sharing its research infrastructures. The authors also acknowledge Istanbul University-Cerrahpasa, Application & Research Center for the Measurement of Food Antioxidants for sharing its research infrastructures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beğiç, N., Bener, M. & Apak, R. Development of a green synthesized silver nanoparticle-based antioxidant capacity method using carob extract. J Nanostruct Chem 11, 381–394 (2021). https://doi.org/10.1007/s40097-020-00374-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-020-00374-6