Abstract
Key message
Exogenous ABA increased the flowering rate and advanced the flower formation of “Fuji” apple cv. “Nagafu No.2” by promoting the accumulation of soluble sugar, glucose and sucrose, and also increased the activities of α-AMY and SPS.
Abstract
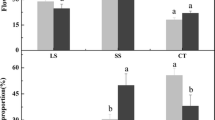
“Fuji” apple (Malus domestica Borkh) has a long vegetative growth phase and difficulty in flower induction, which seriously affects apple production. ABA plays important roles in the flower bud induction of fruit trees. However, the effect of exogenous ABA on flower bud induction of apple trees has rarely been reported. In this experiment, 2-year-old “Fuji” apple cv. “Nagafu No. 2” scion material was grafted on dwarf self-rooted rootstock M.26 (DSR), vigorous rootstock M. sieversii (VR) and interstock M.26/M. sieversii (IR). The control trees were sprayed with water solution, while 100 µmol·L−1 ABA and 5 mmol·L−1 sodium tungstate (ST) were applied as treatments. The results showed that exogenous ABA promoted flower formation and increased the flowering rate, but the impact on the VR was not obvious. Furthermore, the accumulation of soluble sugar, glucose and sucrose, as well as the activities of α-amylase (α-AMY) and sucrose phosphate synthase (SPS) in the leaves and buds were also significantly promoted. However, the starch concentrations and sucrose synthase (SS) activities decreased. The increase of leaf specific weight and decrease of the growth of single leaf area were supportive to the growth of flower buds. Linear relationships were discovered between starch concentration and α-AMY activity (R2 = 0.998, p < 0.05), sucrose concentration and the activities of SS and SPS (R2 = 0.854–0.999, p < 0.05). Besides, exogenous ST had negative effects compared with ABA. Exogenous ABA increased sugar metabolism, and enzyme activities of DSR, and also enhanced the absorption and utilization of sugar substances, resulting in easier flowering in the DSR, followed by the IR, except for the difficult-flowering rootstock VR. Finally, the expression levels of TPS7, PPC1 and ABR1 were up-regulated in leaves and buds after ABA application, while the floral inhibiting gene TFL1 was down-regulated, indicating that exogenous ABA may promote flower bud differentiation by inhibiting TFL1 expression in apple.





Similar content being viewed by others
References
Costes E, García-Villanueva E (2007) Clarifying the effects of dwarfing rootstock on vegetative and reproductive growth during tree development: a study on apple, trees. Ann Bot 100(2):347
Covington ED, Roitsch T, Dermastia M (2016) Determination of the activity signature of key carbohydrate metabolism enzymes in phenolic-rich grapevine tissues. Acta Chim Slov 63(4):757–762
Cui Z, Zhou B, Zhang Z, Hu Z (2013) Abscisic acid promotes flowering and enhances LcAP1 expression in Litchi chinensis Sonn. S Afr J Bot 88:76–79
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Eshghi S, Tafazoli E, Dokhani S, Rahemi M, Emam Y (2007) Changes in carbohydrate concentrations in shoot tips, leaves and roots of strawberry (Fragaria × ananassa Duch.) during flower-bud differentiation. Sci Hortic 113(3):255–260
Fan S, Zhang D, Gao C, Zhao M, Wu H, Li Y, Shen Y, Han M (2017) Identification, classification, and expression analysis of GRAS gene family in Malus domestica. Front Physiol 8:1–19
Flachowsky H, Szankowski I, Waidmann S, Peil A, Tränkner C, Hanke MV (2012) The MdTFL1 gene of apple (Malus× domestica Borkh.) reduces vegetative growth and generation time. Tree physiol 32(10):1288–1301
Fresneau C, Ghashghaie J, Cornic G (2007) Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): role of leaf internal CO2. J Exp Bot 58(11):2983
Hennerty MJ, Forshey CG (1971) Effects of defruiting, scoring, defoliation and shading on the carbohydrate concentration of ‘Golden Delicious’ apple trees. J Pomold Horticult Sci 46(2):153–161
Hill-Cottingham DG, Williams RR (1967) Effect of time of application of fertilizer nitrogen on the growth, flower development and fruit set of maiden apple trees, var. Lord Lambourne, and on the distribution of total nitrogen within the trees. J Pomol Horticult Sci 42(4):319–338
Hisamatsu T, King RW (2008) The nature of floral signals in Arabidopsis. II. Roles for FLOWERING LOCUS T (FT) and gibberellin. J Exp Bot 59(14):3821–3829
Ito A, Hayama H, Kashimura Y (2002) Sugar metabolism in buds during flower bud formation: a comparison of two Japanese pear [Pyrus pyrifolia, (Burm.) Nak.] cultivars possessing different flowering habits. Sci Hortic 96(1):163–175
Kong L, Abrams SR, Owen SJ, Van NA, Von AP (2009) Dynamic changes in concentrations of auxin, cytokinin, ABA and selected metabolites in multiple genotypes of Douglas-fir (Pseudotsuga menziesii) during a growing season. Tree Physiol 29(2):183–190
Koshita Y, Takahara T, Ogata T, Goto A (1999) Involvement of endogenous plant hormones (IAA, ABA, GAS) in leaves and flower bud formation of satsuma mandarin (Citrus unshiu, Marc.). Sci Hortic 79(3–4):185–194
Kotoda N, Wada M (2005) MdTFL1, a TFL1-like gene of apple, retards the transition from the vegetative to reproductive phase in transgenic Arabidopsis. Plant Sci 168(1):95–104
Lee HS, Milborrow BV (1997) Endogenous Biosynthetic Precursors of (+)-Abscisic Acid. V. Inhibition by Tungstate and its Removal by Cinchonine shows that Xanthoxal is Oxidised by a Molybdo-Aldehyde Oxidase. Funct Plant Biol 24(6):727–732
Leng P, Zhang Y, Du Y, Wang J, Jiang L, Kai W, Liang B, Zhai X, Sun Y, Liu H, Wu X, Cheng J, Zhang L (2018) Expression pattern of ABA metabolic and signalling genes during floral development and fruit set in sweet cherry. Plant Growth Regul 84:71–80
Li YM, Zhang D, Xing LB, Zhang SW, Zhao CP, Han MY (2016) Effect of exogenous 6-benzylaminopurine (6-BA) on branch type, floral induction and initiation, and related gene expression in ‘Fuji’ apple (Malus domestica Borkh). Plant Growth Regul 79:65–70
Li Y, Zhang D, Zhang L, Zuo X, Fan S, Zhang X, Shalmani A, Han M (2017) Identification and expression analysis of cytokinin response-regulator genes during floral induction in apple ( Malus domestica Borkh). Plant Growth Regul 83(3):1–10
Li WF, Mao J, Li XW, Su J, Dawuda MM, Ma ZH, Zuo CW, An ZS (2018) Effects of CEPA and 1-MCP on flower bud differentiation of apple cv. ‘Nagafu No. 2’ grafted on different rootstocks. J Plant Growth Regul. https://doi.org/10.1007/s00344-018-9895-7
Liu T, Hu YQ, Li XX (2008) Comparison of dynamic changes in endogenous hormones and sugars between abnormal and normal Castanea mollissima. Prog Nat Sci-Mater 18:685–690
Liu Y, Zhang HP, Gu C, Tao ST, Wang DS, Guo XP, Qi JK, Zhang SL (2016) Transcriptome profiling reveals differentially expressed genes associated with wizened flower bud formation in Chinese pear (Pyrus bretschneideri Rehd). J Hortic Sci Biotec 91(3):227–235
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Ma X, Ma F, Mi Y, Ma Y, Shu H (2008) Morphological and physiological responses of two contrasting Malus, species to exogenous abscisic acid application. Plant Growth Regul 56(1):77–87
Marini RP, Barden JA (1981) Seasonal correlations of specific leaf weight to net photosynthesis and dark respiration of apple leaves. Photosynth Res 2:251–258
McCreddy RM, Guggolz J, Silviera V, Owens HS (1950) Determination of starch and amylase in vegetables. Anal Chem 22:1156–1158
Mimida N, Li J, Zhang C, Moriya S, Moriya-Tanaka Y, Iwanami H, Honda C, Oshino H, Takagishi K, Suzuki A, Komori S, Wada M (2012) Divergence of terminal flower1-like genes in Rosaceae. Biol Plantarum 56(3):465–472
Mir R, Hernández ML, Abou-Mansour E, Martínez-Rivas JM, Mauch F, Métraux JP, León J (2013) Pathogen and Circadian Controlled 1 (PCC1) regulates polar lipid concentration, ABA-related responses, and pathogen defence in Arabidopsis thaliana. J Exp Bot 64(11):3385–3395
Murcia G, Pontin M, Reinoso H, Baraldi R, Bertazza G, Gómeztalquenca S, Gómez-Talquenca S, Bottini R, Piccoli PN (2016) ABA and GA3 increase carbon allocation in different organs of grapevine plants by inducing accumulation of non-structural carbohydrates in leaves, enhancement of phloem area and expression of sugar transporters. Physiol Plantarum 156(3):323–337
Pandey GK, Grant JJ, Yong HC, Li L, Sheng L (2005) ABR1, an APETALA2-domain transcription factor that functions as a repressor of ABA response in Arabidopsis. Plant Physiol 139(3):1185
Porri A, Torti S, Romera-Branchat M, Coupland G (2012) Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods. Development 139:2198–2209
Purgatto E, Lajolo FM, Cordenunsi BR (2001) Inhibition of β-amylase activity, starch degradation and sucrose formation by indole-3-acetic acid during banana ripening. Planta 212(5):823–828
Samad A, Mcneil DL, Khan ZU (1999) Effect of interstock bridge grafting (M9 dwarfing rootstock and same cultivar cutting) on vegetative growth, reproductive growth and carbohydrate composition of mature apple trees. Sci Hortic 79(1–2):23–38
Segarra S, Mir R, Martínez C, León J (2010) Genome-wide analyses of the transcriptomes of salicylic acid-deficient versus wild type plants uncover Pathogen and Circadian Controlled 1 (PCC1) as a regulator of flowering time in Arabidopsis. Plant Cell Environ 33:11–22
Seung D, Risopatron JPM, Jones BJ, Marc J (2012) Circadian clock-dependent gating in ABA signalling networks. Protoplasma 249:445–457
Shuster L, Gifford RH (1962) Changes in nucleotidases during the germination of wheat embryo. Arch Biochem Biophys 96:532–540
Smeekens S, Ma J, Hanson J, Rolland F (2010) Sugar signals and molecular networks controlling plant growth. Curr Opin Plant Biol 13(3):273–278
Strand A, Zrenner R, Trevanion S, Stitt M, Gustafsson P, Gardeström P (2000) Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1,6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J 23(6):759–770
Sun L, Chen W, Meng Y, Yang X, Yuan L, Guo Y (2016) Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chem 208:51–60
Tanase K, Shiratake K, Mori H, Yamaki S (2002) Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol Plantarum 114(1):21–26
Tworkoski T, Miller S (2007) Endogenous hormone concentrations and bud-break response to exogenous benzyl adenine in shoots of apple trees with two growth habits grown on three rootstocks. J Hortic Sci Biotechn 82(6):960–966
Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339(6120):704–707
Wunsche JN, Greer DH, Laing WA, Palmer JW (2005) Physiological and biochemical leaf and tree responses to crop load in apple. Tree Physiol 25(10):1253–1263
Xi W, Liu C, Hou X, Yu H (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 22:1733–1748
Xing LB, Zhang D, Li YM, Zhao CP, Zhang SW, Shen YW, An N, Han MY (2014) Genome-wide identification of vegetative phase transition-associated microRNAs and target predictions using degradome sequencing in Malus hupehensis. BMC Genomics 15:1125
Xing LB, Zhang D, Li YM, Shen YW, Zhao CP, Ma JJ, An N, Han MY (2015) Transcription profiles reveal sugar and hormone signaling pathways mediating flower induction in apple (Malus domestica Borkh.). Plant Cell Physiol 56:2052–2068
Xing L, Zhang D, Zhao C, Li Y, Ma J, An N, Han M (2016) Shoot bending promotes flower bud formation by miRNA-mediated regulation in apple (Malus domestica Borkh.). Plant Biotechnol J 14(2):749–770
Zhang SW, Zhang D, Fan S, Du L, Shen Y, Xing L, Li Y, Ma J, Han M (2016) Effect of exogenous GA3 and its inhibitor paclobutrazol on floral formation, endogenous hormones, and flowering-associated genes in ‘Fuji’ apple (Malus×domestica Borkh.). Plant Physiol Bioch 107:178–186
Zhu G, Ye N, Yang J, Peng X, Zhang J (2011) Regulation of expression of starch synthesis genes by ethylene and ABA in relation to the development of rice inferior and superior spikelets. J Exp Bot 62(11):3907–3916
Acknowledgements
This research was financially supported by the grants from the Discipline Construction Fund Project of Gansu Agricultural University (GSAU-XKJS-2018-230), the Science and Technology Major Project of Gansu Province (18ZD2NA006), the College Industry Support and Guidance Project of Gansu Province (2019C11), and the Fostering Foundation for the Excellent Ph.D. Dissertation of Gansu Agricultural University (2017002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Communicated by Magel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, WF., Mao, J., Su, J. et al. Exogenous ABA and its inhibitor regulate flower bud induction of apple cv. ‘Nagafu No. 2′ grafted on different rootstocks. Trees 35, 609–620 (2021). https://doi.org/10.1007/s00468-020-02063-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-020-02063-x