Abstract
Key message
StMAPK11 overexpression promotes potato growth, physiological activities and photosynthesis under drought conditions.
Abstract
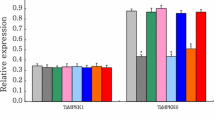
Mitogen-activated protein kinases (MAPKs) are import regulators of MAPK pathway in plants under drought condition. However, the critical role in potato (Solanum tuberosum L.) drought resistance is not fully understood. In this study, we aimed to explore the role of StMAPK11 under drought stress. The result of RT-qPCR for assay of StMAPKs expression demonstrated that 15 StMAPKs were differentially expressed in leaves, flowers, petioles, stamens, pistils, stems, stolons, roots, tubers and tuber peels of potato. StMAPKs was dynamically modulated by abiotic stresses and plant hormone treatments, and StMAPK11 was apparently up-regulated under drought conditions. Therefore, the vectors pCPB-StMAPK11 and pCPBI121-miRmapk11 for over-expression and down-regulation of StMAPK11 were constructed, respectively, and introduced into potato cultivar Atlantic. The result showed that StMAPK11 promoted potato growth under drought conditions, as well as the physiological activities evidenced by changes in SOD, CAT and POD activity and H2O2, proline and MDA content. StMAPK11 up-regulation intensified drought resistance of potato plant by elevating antioxidant activities and photosynthesis. Moreover, we consolidated the protective role of StMAPK11 in tobacco and Arabidopsis against drought stress. The result could provide new insights into the function of StMAPK11 in drought response and its possible mechanisms.







Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Agrawal GK, Agrawal SK, Shibato J, Iwahashi H, Rakwal R (2003) Novel rice MAP kinases OsMSRMK3 and OsWJUMK1 involved in encountering diverse environmental stresses and developmental regulation. Biochem Biophys Res Commun. https://doi.org/10.1016/s0006-291x(02)02868-1
Andrasi N, Rigo G, Zsigmond L, Perez-Salamo I, Papdi C, Klement E, Pettko-Szandtner A, Baba AI, Ayaydin F, Dasari R, Cseplo A, Szabados L (2019) The mitogen-activated protein kinase 4-phosphorylated heat shock factor A4A regulates responses to combined salt and heat stresses. J Exp Bot 70:4903–4918
Anjum SA, Ashraf U, Tanveer M, Khan I, Hussain S, Shahzad B, Zohaib A, Abbas F, Saleem MF, Ali I, Wang LC (2017) Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front Plant Sci 8:69–69. https://doi.org/10.3389/fpls.2017.00069
Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI (2019) Genetic strategies for improving crop yields. Nature 575:109–118
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bigeard J, Hirt H (2018) Nuclear signaling of plant MAPKs. Front Plant Sci 9:469. https://doi.org/10.3389/fpls.2018.00469
Bouaziz D, Charfeddine M, Jbir R, Najib Saidi M, Pirrello J, Charfeddine S, Bouzayen M, Gargouri-Bouzid R (2015) Identification and functional characterization of ten AP2/ERF genes in potato. Plant Cell Tissue Organ Culture 123:155–172. https://doi.org/10.1007/s11240-015-0823-2
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Cai G, Wang G, Wang L, Liu Y, Pan J, Li D (2014) A maize mitogen-activated protein kinase kinase, ZmMKK1, positively regulated the salt and drought tolerance in transgenic Arabidopsis. J Plant Physiol 171:1003–1016
Chardin C, Schenk ST, Hirt H, Colcombet J, Krapp A (2017) Review: mitogen-activated protein kinases in nutritional signaling in Arabidopsis. Plant Sci 260:101–108
Chen L, Sun H, Wang F, Yue D, Shen X, Sun W, Zhang X, Yang X (2020) Genome-wide identification of MAPK cascade genes reveals the GhMAP3K14–GhMKK11–GhMPK31 pathway is involved in the drought response in cotton. Plant MolBiol 103:211–223. https://doi.org/10.1007/s11103-020-00986-0
Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK (2007) Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiol 145:890–904. https://doi.org/10.1104/pp.107.103325
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. https://doi.org/10.1046/j.1365-313x.1998.00343.x
Dai G, Peng K, Xiao L, Deng G (2006) Effect of drought stress simulated by PEG on malonaldehyde, proline contents and superoxide dismutase activity in low potassium tolerant rice seedlings. Zhongguo Shuidao Kexue 20:557–559
Doczi R, Brader G, Pettko-Szandtner A, Rajh I, Djamei A, Pitzschke A, Teige M, Hirt H (2007) The Arabidopsis mitogen-activated protein kinase kinase MKK3 is upstream of group C mitogen-activated protein kinases and participates in pathogen signaling. Plant Cell 19:3266–3279. https://doi.org/10.1105/tpc.106.050039
Edwards K, Johnstone C, Thompson C (1991) A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19:1349. https://doi.org/10.1093/nar/19.6.1349
Gashaw B, Mohammed W, Tesfaye A (2018) Genetic diversity in drought tolerant Potato (Solanum tuberosum L.) genotypes in Simada North western Ethiopia. J Agric Environ Int Dev 112:121–138. https://doi.org/10.12895/jaeid.20181.720
Guo T, Chen K, Dong NQ, Shi CL, Ye WW, Gao JP, Shan JX, Lin HX (2018) GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell Rep 30:871–888. https://doi.org/10.1105/tpc.17.00959
Hamel LP, Nicole MC, Sritubtim S, Morency MJ, Ellis M, Ehlting J, Beaudoin N, Barbazuk B, Klessig D, Lee J, Martin G, Mundy J, Ohashi Y, Scheel D, Sheen J, Xing T, Zhang S, Seguin A, Ellis BE (2006) Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci 11:192–198. https://doi.org/10.1016/j.tplants.2006.02.007
Hao L, Wen Y, Zhao Y, Lu W, Xiao K (2015) Wheat mitogen-activated protein kinase gene TaMPK4 improves plant tolerance to multiple stresses through modifying root growth ROS metabolism, and nutrient acquisitions. Plant Cell Rep 34:2081–2097. https://doi.org/10.1007/s00299-015-1853-2
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hussain S, Khalid MF, Saqib M, Ahmad S, Zafar W, Rao MJ, Morillon R, Anjum MA (2018) Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiol Plant 40:135
Iftikhar H, Naveed N, Virk N, Bhatti MF, Song F (2017) In silico analysis reveals widespread presence of three gene families MAPK, MAPKK and MAPKKK, of the MAPK cascade from crop plants of Solanaceae in comparison to the distantly-related syntenic species from Rubiaceae, coffee. PeerJ 5:e3255. https://doi.org/10.7717/peerj.3255
Jagodzik P, Tajdel-Zielinska M, Ciesla A, Marczak M, Ludwikow A (2018) Mitogen-activated protein kinase cascades in plant hormone signaling. Front Plant Sci 9:1387. https://doi.org/10.3389/fpls.2018.01387
Kazerani B, Navabpour S, Sabouri H, Ramezanpour SS, Zaynali Nezhad K, Eskandari A (2019) Evaluation of proline content and enzymatic defense mechanism in response to drought stress in rice. Plant Physiol 9:2749–2757. https://doi.org/10.22034/ijpp.2019.664580
Kim HS, Park S-C, Ji CY, Park S, Jeong JC, Lee H-S, Kwak S-S (2016) Molecular characterization of biotic and abiotic stress-responsive MAP kinase genes, IbMPK3 and IbMPK6, in sweetpotato. Plant Physiol Biochem 108:37–48
Kong X, Sun L, Zhou Y, Zhang M, Liu Y, Pan J, Li D (2011) ZmMKK4 regulates osmotic stress through reactive oxygen species scavenging in transgenic tobacco. Plant Cell Rep 30:2097. https://doi.org/10.1007/s00299-011-1116-9
Lau KH, Del Rosario HM, Crisovan E, Wu S, Fei Z, Khan MA, Buell CR, Gemenet DC (2018) Transcriptomic analysis of sweet potato under dehydration stress identifies candidate genes for drought tolerance. Plant Direct 2:e00092. https://doi.org/10.1002/pld3.92
Lee CC, Wu YJ, Hsueh CH, Huang YT, Hsu YH, Meng M (2018) Mitogen-activated protein kinase phosphatase 1 reduces the replication efficiency of Bamboo mosaic virus in Nicotiana benthamiana. Mol Plant Pathol 19:2319–2332. https://doi.org/10.1111/mpp.12701
Li H (2000) Determination of superoxide dismutase activity by the means of nitroblue tetrazolium. In: Principles and techniques of plant physiological biochemical experiments. Higher Education Press, Beijing, p 293
Li JB, Luan YS, Liu Z (2015) Overexpression of SpWRKY1 promotes resistance to Phytophthora nicotianae and tolerance to salt and drought stress in transgenic tobacco. Physiol Plant 155:248–266. https://doi.org/10.1111/ppl.12315
Liu H, Weisman D, Tang L, Tan L, Zhang W-k, Wang Z-h, Huang Y-h, Lin W-x, Liu X-m, Colón-Carmona A (2015a) Stress signaling in response to polycyclic aromatic hydrocarbon exposure in Arabidopsis thaliana involves a nucleoside diphosphate kinase, NDPK-3. Planta 241:95–107. https://doi.org/10.1007/s00425-014-2161-8
Liu S, Hua L, Dong S, Chen H, Zhu X, Jiang J, Zhang F, Li Y, Fang X, Chen F (2015b) OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J 84:672–681. https://doi.org/10.1111/tpj.13025
Long L, Gao W, Xu L, Liu M, Luo X, He X, Yang X, Zhang X, Zhu L (2014) GbMPK3, a mitogen-activated protein kinase from cotton, enhances drought and oxidative stress tolerance in tobacco. Plant Cell Tissue Organ Culture 116:153–162. https://doi.org/10.1007/s11240-013-0392-1
López-Bucio JS, Dubrovsky JG, Raya-González J, Ugartechea-Chirino Y, López-Bucio J, de Luna-Valdez LA, Ramos-Vega M, León P, Guevara-García AA (2014) Arabidopsis thaliana mitogen-activated protein kinase 6 is involved in seed formation and modulation of primary and lateral root development. J Exp Bot 65:169–183. https://doi.org/10.1093/jxb/ert368
Mathobo R, Marais D, Steyn JM (2017) The effect of drought stress on yield, leaf gaseous exchange and chlorophyll fluorescence of dry beans (Phaseolus vulgaris L.). Agric Water Manage 180:118–125
Meise P, Seddig S, Uptmoor R, Ordon F, Schum A (2019) Assessment of yield and yield components of starch potato cultivars (Solanum tuberosum L.) under nitrogen deficiency and drought stress conditions. Potato Res 62:193–220. https://doi.org/10.1007/s11540-018-9407-y
Mi X, Liu X, Yan H, Liang L, Zhou X, Yang J, Si H, Zhang N (2017) Expression of the Galanthus nivalis agglutinin (GNA) gene in transgenic potato plants confers resistance to aphids. Comptes Rendus Biol 340:7–12. https://doi.org/10.1016/j.crvi.2016.10.003
Mohammed W, Burga S (2015) Evaluation of potato (Solanum tuberosum L.) genotypes for yeild and tuber quality related traits at Lowland, Dire Dawa, Eastern Ethiopia. Sci, Technol Arts Res J 4:1–10. https://doi.org/10.4314/star.v4i3.1
Mohanta TK, Arora PK, Mohanta N, Parida P, Bae H (2015) Identification of new members of the MAPK gene family in plants shows diverse conserved domains and novel activation loop variants. BMC Genomics 16:58
Muhammad T, Zhang J, Ma Y, Li Y, Zhang F, Zhang Y, Liang Y (2019) Overexpression of a mitogen-activated protein kinase SlMAPK3 positively regulates tomato tolerance to cadmium and drought stress. Molecules 24:556
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Ortiz-Masia D, Perez-Amador MA, Carbonell J, Marcote MJ (2007) Diverse stress signals activate the C1 subgroup MAP kinases of Arabidopsis. FEBS Lett 581:1834–1840. https://doi.org/10.1016/j.febslet.2007.03.075
Ortiz-Masia D, Perez-Amador MA, Carbonell P, Aniento F, Carbonell J, Marcote MJ (2008) Characterization of PsMPK2, the first C1 subgroup MAP kinase from pea (Pisum sativum L.). Planta 227:1333–1342. https://doi.org/10.1007/s00425-008-0705-5
Pan J, Zhang M, Kong X, Xing X, Liu Y, Zhou Y, Liu Y, Sun L, Li D (2012) ZmMPK17, a novel maize group D MAP kinase gene, is involved in multiple stress responses. Planta 235:661–676. https://doi.org/10.1007/s00425-011-1510-0
Rehman N, Khan MR, Abbas Z, Rafique RS, Zaynab M, Qasim M, Noor S, Inam S, Ali GM (2020) Functional characterization of Mitogen-Activated Protein Kinase Kinase (MAPKK) gene in Halophytic Salicornia europaea against salt stress. Environ Exp Bot 171:103934
Rohila JS, Yang Y (2007) Rice mitogen-activated protein kinase gene family and its role in biotic and abiotic stress response. J Integr Plant Biol 49:751–759. https://doi.org/10.1111/j.1672-9072.2007.00501.x
Rudack K, Seddig S, Sprenger H, Köhl K, Uptmoor R, Ordon F (2017) Drought stress-induced changes in starch yield and physiological traits in potato. J Agron Crop Sci 203:494–505. https://doi.org/10.1111/jac.12224
Shi J, An HL, Zhang LA, Gao Z, Guo XQ (2010) GhMPK7, a novel multiple stress-responsive cotton group C MAPK gene, has a role in broad spectrum disease resistance and plant development. Plant Mol Biol 74:1–17. https://doi.org/10.1007/s11103-010-9661-0
Si H-J, Xie C-H, Liu J (2003) An efficient protocol for Agrobacterium-mediated transformation with microtuber and the introduction of an antisense class I patatin gene into potato. Acta Agronomica Sinica 29:801–805
Sinha AK, Jaggi M, Raghuram B, Tuteja N (2011) Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav 6:196–203. https://doi.org/10.4161/psb.6.2.14701
Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1:2019–2025. https://doi.org/10.1038/nprot.2006.286
Taj G, Agarwal P, Grant M, Kumar A (2010) MAPK machinery in plants: recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav 5:1370–1378. https://doi.org/10.4161/psb.5.11.13020
Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15:141–152. https://doi.org/10.1016/j.molcel.2004.06.023
Thomas GM, Huganir RL (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5:173–183. https://doi.org/10.1038/nrn1346
Tian S, Mao X, Zhang H, Chen S, Zhai C, Yang S, Jing R (2013) Cloning and characterization of TaSnRK2.3, a novel SnRK2 gene in common wheat. J Exp Bot 64:2063–2080. https://doi.org/10.1093/jxb/ert072
Wang L, Liu Y, Cai G, Jiang S, Pan J, Li D (2014) Ectopic expression of ZmSIMK1 leads to improved drought tolerance and activation of systematic acquired resistance in transgenic tobacco. J Biotechnol 172:18–29. https://doi.org/10.1016/j.jbiotec.2013.11.006
Wang L, Liu Y, Feng S, Yang J, Li D, Zhang J (2017) Roles of plasmalemma aquaporin gene stpip1 in enhancing drought tolerance in potato. Front Plant Sci 8:616. https://doi.org/10.3389/fpls.2017.00616
Wang Y, Schuck S, Wu J, Yang P, Doring AC, Zeier J, Tsuda K (2018) A MPK3/6-WRKY33-ALD1-pipecolic acid regulatory loop contributes to systemic acquired resistance. Plant Cell 30:2480–2494. https://doi.org/10.1105/tpc.18.00547
Wimalasekera R, Scherer GFE (2018) Chapter 21—Involvement of mitogen-activated protein kinases in abiotic stress responses in plants. In: Ahmad P, Ahanger MA, Singh VP, Tripathi DK, Alam P, Alyemeni MN (eds) Plant metabolites and regulation under environmental stress. Academic Press, pp 389–395
Wu L, Zu X, Zhang H, Wu L, Xi Z, Chen Y (2015) Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol Biol 88:429–443. https://doi.org/10.1007/s11103-015-0333-y
Xiong L, Yang Y (2003) Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell 15:745–759. https://doi.org/10.1105/tpc.008714
Xu R, Duan P, Yu H, Zhou Z, Zhang B, Wang R, Li J, Zhang G, Zhuang S, Lyu J, Li N, Chai T, Tian Z, Yao S, Li Y (2018) Control of grain size and weight by the OsMKKK10-OsMKK4-OsMAPK6 signaling pathway in rice. Mol Plant 11:860–873. https://doi.org/10.1016/j.molp.2018.04.004
Zaheer K, Akhtar MH (2016) Potato production, usage, and nutrition–a review. Crit Rev Food Sci Nutr 56:711–721. https://doi.org/10.1080/10408398.2012.724479
Zhang Z, Li J, Li F, Liu H, Yang W, Chong K, Xu Y (2017) OsMAPK3 phosphorylates OsbHLH002/OsICE1 and inhibits its ubiquitination to activate OsTPP1 and enhances rice chilling tolerance. Dev Cell 43:731-743.e735. https://doi.org/10.1016/j.devcel.2017.11.016
Zhang M, Su J, Zhang Y, Xu J, Zhang S (2018) Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr Opin Plant Biol 45:1–10. https://doi.org/10.1016/j.pbi.2018.04.012
Zhou X, Zhang N, Yang J, Tang X, Wen Y, Si H (2018) Functional analysis of StDWF4 gene in response to salt stress in potato. Plant Physiol Biochem 125:63–73. https://doi.org/10.1016/j.plaphy.2018.01.027
Zhu X, Zhang N, Liu X, Wang S, Li S, Yang J, Wang F, Si H (2020a) StMAPK3 controls oxidase activity, photosynthesis and stomatal aperture under salinity and osmosis stress in potato. Plant PhysiolBiochem 156:167–177
Zhu D, Chang Y, Pei T, Zhang X, Liu L, Li Y, Zhuang J, Yang H, Qin F, Song C, Ren D (2020b) MAPK-like protein 1 positively regulates maize seedling drought sensitivity by suppressing ABA biosynthesis. Plant J 102:747–760. https://doi.org/10.1111/tpj.14660
Acknowledgements
We thank Rongkai Wang (Bioediates Technology Corporation, Shanxi, China) for providing pART-CAM and pCAM35S-GFP expression vector.
Funding
This Research Program Sponsored by Gansu Provincial Key Laboratory of Aridland Crop Science, Gansu Agricultural University (No.GSCS-2019-Z03) and National Natural Science Foundation of China (No.31960444).
Author information
Authors and Affiliations
Contributions
XZ, NZ and HS planned and designed the research. XZ, XL, SL, JY, XH and FW, collected the data. XZ, NZ, XL, SL and JY analyzed the data. XZ, NZ, XL and HS drafted the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
299_2020_2645_MOESM4_ESM.tif
Supplementary Fig. 1 The antigen and antibody were prepared using Escherichia coli BL-21 strain and rabbit. PAGE analysis and Coomassie brilliant blue staining were used to detect (a) expression of recombinant protein StMAPK11. M, protein marker; 1, Protein extract from BL-21 stain without transfection. 2, Protein extract from the transfected BL-21 without IPTG induction; 3, IPTG induction at 16℃; 4, IPTG induction with 8 mol/L urea denaturation at 16oC; 5, IPTG induction at 30℃; 6, IPTG induction with 8 mol/L urea denaturation at 30℃; 7, IPTG induction at 37℃; 8, IPTG induction with 8 mol/L urea denaturation at 37℃; (b) affinity purification of recombinant protein StMAPK11. M, protein marker; 1, Antigen from BL-21 transfected with StMAPK11 with IPTG induction at 37℃ and denaturation in 4 mol/L urea. (c) WB verification of antibodies. M, Protein marker; 1-3, serum antibody titer is 1:32,000, and sample volume is 0.5 μg, 1 μg and 2 μg, respectively; 4-6, serum antibody titer is 1:64,000, and sample volume is 0.5 μg, 1 μg and 2 μg, respectively (TIF 997 KB)
299_2020_2645_MOESM5_ESM.tif
Supplementary Fig. 2 Potato plants were transfected with the recombinant plasmids designed for down-regulating or up-regulating StMAPK11 mRNA. PCR products were amplified from the plants (Lane 1-13) transformed with the recombinant plasmids (a) pCPB-StMAPK11 and (b) pCPBI121-miRmapk11 carrying NPT-II gene. M, DNA molecular weight standard DL2000; P, positive control; NT, non-transformed control; CK, control; NPT-II, 676 bp (TIF 7010 KB)
299_2020_2645_MOESM6_ESM.tif
Supplementary Fig. 3 Nicotiana tabacum (tobacco) and Arabidopsis thaliana (Arabidopsis) were transformed with the recombinant plasmids designed for up-regulating StMAPK11. PCR products were synthesized from (a) tobacco (Lane 1-16) and (b) Arabidopsis (Lane 1-13) transformed with pART-CAM-StMAPK11 plasmid. M, DNA molecular weight standard DL2000; P, positive control; WT, wild type; CK, control; PCR products, 415 bp and 728 bp (TIF 6947 KB)
Rights and permissions
About this article
Cite this article
Zhu, X., Zhang, N., Liu, X. et al. Mitogen-activated protein kinase 11 (MAPK11) maintains growth and photosynthesis of potato plant under drought condition. Plant Cell Rep 40, 491–506 (2021). https://doi.org/10.1007/s00299-020-02645-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02645-6