Abstract
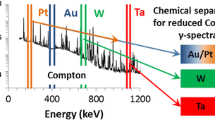
The use of fluorination as a rapid separation technique for quantification of trace-mass, short-lived isotopes is illustrated. Single crystal 238UO2 was exposed to a 14 MeV neutron source to produce mixed fission and activation products. Selective removal of trace volatile fluorides from non-volatile ones produced two results of interest. A decrease in background produced better detection and quantification of select analytes across the gamma spectrum. Second, at the very low burnup condition described, the volatility behaviors of common isotopes were altered relative to their well-established behaviors in higher burnup metal and metal oxide fuels.


Similar content being viewed by others
Notes
MCNP6.2® and Monte Carlo N-Particle® are registered trademarks owned by Triad National Security, LLC, manager and operator of Los Alamos National Laboratory. Any third-party use of such registered marks should be properly attributed to Los Alamos National Security, LLC, including the use of the designation as appropriate. For the purposes of visual clarity, the registered trademark symbol is assumed for all references to MCNP within the remainder of this paper.
References
Morley SM, Seiner B, Finn E, Greenwood LR, Smith SC, Gregory S, Swearingen K (2015) Integrated separation scheme for measuring a suite of fission and activation products from a fresh mixed fission and activation product sample. J Radioanal Nucl Chem 304(2):509–515
Arrigo LM, Greenwood LR, Pierson BD, Metz LA, Friese JI (2018) Radiochemical separations and experimental measurements of short-lived fission products from 14 MeV irradiation of depleted uranium. J Radioanal Nucl Chem 318(1):353–360
Morrison SS, Clark SB, Eggemeyer TA, Finn EC, Hines CC, King MD, Seiner BN (2017) Activation product analysis in a mixed sample containing both fission and neutron activation products. J Radioanal Nucl Chem 314(3):2501–2506
Haney MM, Seiner BN, Finn EC, Friese JI (2016) Rapid quantitation of uranium from mixed fission product samples. J Radioanal Nucl Chem 307(3):1737–1742
Morrison SS, Seiner BN, Eggemeyer TA, Haney MM, Hines CC, King MD, Zhang Z (2016) A chemical separation procedure using ionic liquid extraction for 59 Fe and 55 Fe quantification. J Radioanal Nucl Chem 307(3):2479–2485
Morrison SS, Morrison EC, Uhnak NE, Wittman RS, Seiner BN (2019) Isolation of Au and Pt radionuclides from deuteron-irradiated platinum using TBP resin. J Radioanal Nucl Chem 319(3):679–686
Bennett KT, Kozimor SA, Manard BT, Mocko V, Pacheco SD, Schake AR, Olson AC (2019) Rapid activation product separations from fission products and soil matrixes. J Radioanal Nucl Chem 322(2):281–289
Dry DE, Bauer E, Petersen LA (2005) Rapid separation of fresh fission products. J Radioanal Nucl Chem 263(1):19–22
Fan J, Lu J, Zhang L, Yu G, Huang P, Shi Q, Bai T (2016) Automated separation of short-lived 72 Ga from fresh fission products based on tandem column chromatography. J Radioanal Nucl Chem 309(2):467–476
Jonke A (1965) Reprocessing of nuclear reactor fuels by processes based on volatilization fractional distillation, and selective adsorption. Atomic Energy Rev 3:3–60
Brater DC, Kaufman HL, Pashley JH, Smiley SH (1966) Removal of impurities from uranium hexafluoride by selective sorption techniques. AEC Research and Development Union Carbide Corporation, Report K-1666
Shatalov V, Seregin M, Kharin V, Ponomarev L (2001) Gas-fluoride technology for processing spent oxide fuel. At Energ 90(3):224–234
Chilenskas AA (1968) Fluidized-bed fluoride volatility processing of irradiated UO2 Fuels. Nucl Appl 5(1):11–19
Schmets JJ (1970) Reprocessing of spent nuclear fuels by volatility processes. Atomic Energy Rev 8:3–60
Scott CD, Carter WL (1966) Preliminary design study of a continuous fluorination-vacuum distillation system for regenerating fuel and fertile streams in a molten salt breeder reactor. ORNL-3791, Oak Ridge National Laboratory Oak Ridge Tennessee
Carr WH, King LJ, Kitts FG, McDuffee WT, Miles FW (1966) Molten salt fluoride volatility pilot plant: recovery of enriched uranium from aluminum-clad fuel elements. Oak Ridge National Laboratory, Oak Ridge, Oak Ridge, Tennessee, ORNL-4574
Trevorrow LE, Gerding TJ, Steindler MJ (1969) Neptunium separation from uranium. United States Patent No. 3482949
Weinstock B, Crist RH (1948) The vapor pressure of uranium hexafluoride. J Chem Phys 16:436–441
Weinstock B, Weaver EE, Malm JG (1959) Vapour pressures of NpF6 and PuF6, thermodynamic calculations with UF6, NpF6 and PuF6. J Inorg Nucl Chem 11(2):104–114
McNamara BK, O’Hara MJ, Clark RA, Morrison SS, Soderquist CZ, Scheele RD (2020) Gas-phase molybdenum-99 separation from uranium dioxide by fluoride volatility using nitrogen trifluoride. RSC Adv 10:4175–4188
McNamara BK, Buck EC, Soderquist CZ, Smith F, Mausoulf E, Scheele RD (2014) Separation of metallic residues from the dissolution of a high-burnup BWR fuel using nitrogen trifluoride. J Fluorine Chem 162:1–8
Weber WJ (1981) Ingrowth of lattice defects in alpha irradiated UO2, single crystals. J Nucl Mater 98:206–215
Corbey JF, Reilly DD, Sweet LE, Lach TG (2019) Extraction of plutonium-containing microcrystals from Hanford soil using a focused ion beam for single-crystal x-ray diffraction analysis. J Appl Cryst 52(6):1244–1252
McNamara BK, Clark RA, O’Hara MJ, Morrison SS, Pierson BD, Parker CM, Carter RA (2018) New methods for rapid separation of volatile forensic analytes from post-detonation debris. Pacific Northwest National Laboratory, PNNL-28028
Casella AM, Scheele RD, McNamara BK (2015) Characterization of the kinetics of NF3-fluorination of NpO2. AIP Adv 5(127230):1–16
Sonzogni AA (2005) Nudat 2.0: Nuclear structure and decay data on the internet. AIP Conf Proc 769: 574–577
Brunetti B, Piacente V, Scardala P (2008) Vapor pressures and sublimation enthalpies of copper difluoride and silver (I, II) fluorides by the torsion-effusion method. J Chem Eng Data 53:687–693
Abate L, Wilhelm H, (1951) Sublimation of zirconium tetrafluoride. Ames National Laboratory, ISC-151
Scheele RD, McNamara BK, Casella AM, Kozelisky AE (2012) On the use of thermal NF3 as the fluorination and oxidation agent in the treatment of used nuclear fuels. J Nucl Mater 424:224–236
Henrion P, Leurs A (1971) Kinetics of the reaction of UO2, NpO2 and (U, Pu)O2 with fluorine and its chlorine derivatives. J Nucl Mater 41:1–22
Ogata S, Homma S, Sashira A, Kawamura F, Koga J, Matsumoto S (2004) Fluorination Reaction of Uranium Dioxide by Fluorine. J Nucl Sci Tech 41(2):135–141
McNamara BK, O’Hara MJ (2016) Uniform deposition of uranium hexafluoride (UF6): standardized mass deposits and controlled isotopic ratios using a thermal fluorination method. Talanta 154:219–227
Sakurai T (1974) Comparison the fluorinations of uranium dioxide by bromine trifluoride and elemental fluorine. J Phys Chem 78(12):1140–1144
Hoak SM (2018) Native defect characterization of single crystal UO2 pre- and post-neutron irradiation. Thesis, AFIT-ENP-MS-18-M-084, Air Force Institute of Technology, Wright-Patterson Air Force Base, Ohio
Bendall PJ, Catlow CRA, Corish J, Jacobs PWM (1984) Defect aggregation in anion-excess fluorites II. Clusters containing more than two impurity atoms. J Solid State Chem 51:159–169
Stanek CR (2003) Atomic scale disorder in fluorite and fluorite related oxides. Ph.D. thesis, University of London, Department of Materials Imperial College of Science, Technology and Medicine, London
Abramowski M (2001) Atomistic simulations of the uranium/oxygen system Ph.D. thesis, Imperial College, London
McNamara BK, Scheele RD, Kozelisky AE, Edwards ME (2009) Thermal reactions of uranium metal, UO2, U3O8, UF4, and UO2F2 with NF3 to produce UF6. J Nucl Mater 394:166–173
Sakurai T, Takahashi A (1978) Behavior of ruthenium in fluoride-volatility processes, (IV) Ruthenium species appearing in fluorination step. J Nucl Sci Tech 15(8):574–579
Kleykamp H (1985) The chemical state of the fission products in oxide fuels. J Nucl Mater 131:221–246
Buck EC, Mausolf EJ, McNamara BK, Soderquist CZ, Schwantes JM (2015) Nanostructure of metallic particles in light water reactor used nuclear fuel. J Nucl Mater 461:236–243
Schwantes JM, Bair JL, Buck EC, Devanathan R, Kessler SH, Lach TG, Lonergan JM, McNamara BK, Palmer CJ, Clark RA (2020) A new non-diffusional gas bubble production route in used nuclear fuel: implications for fission gas release, cladding corrosion, and next generation fuel design. Phys Chem Chem Phys 22:6086–6099
Pellegrini KL, Soderquist CZ, Shen SD, Krogstad EJ, Palmer CJ, Gerez K, Buck EC, Lach TG, Schwantes JM, Clark RA (2019) Chemical and isotopic characterization of noble metal phase from commercial UO2 fuel. Anal Chem 91(10):6522–6529
Pomiro F, Gaviría J, Bohé A, De Micco G (2020) Thermodynamic analysis of uranium oxides fluorination with HF(g) and F2(g). J Radioanal Nucl Chem 324:1283–1292
Forbes TZ, Burns PC, Skanthakumar S, Soderholm L (2007) Synthesis, structure, and magnetism of Np2O5. J Am Chem Soc 129:2760–2761
Domanov VP (2009) Formation of volatile neptunium trioxide NpO3 and two other products of neptunium oxidation. Radiochemistry 51(4):350–353
Eller PG, Asprey LB, Kinkead SA, Swanson BI, Kissane RJ (1998) Reactions of dioxygen difluoride with neptunium oxides and fluorides. J Alloys Compds 269(1):63–66
Acknowledgements
This work was performed at Pacific Northwest National Laboratory. The Pacific Northwest National Laboratory is operated for the U.S. Department of Energy by Battelle under contract DE-AC06-67RLO 1830.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McNamara, B.K., Morrison, S. & Pierson, B. Potential defect structure effects in the volatility separation of sub-picogram quantities of fission and activation products from an irradiated UO2 matrix. J Radioanal Nucl Chem 327, 21–30 (2021). https://doi.org/10.1007/s10967-020-07512-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-020-07512-y