Abstract
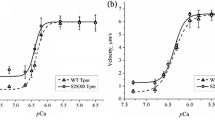
Phosphorylation of α-tropomyosin (Tpm1.1), a predominant Tpm isoform in the myocardium, is one of the regulatory mechanisms of the heart contractility. The Tpm 1.1 molecule has one site of phosphorylation, Ser283. The degree of the Tpm phosphorylation decreases with age and also changes in heart pathologies. Myocardial pathologies, in particular ischemia, are usually accompanied by pH lowering in the cardiomyocyte cytosol. We studied the effects of acidosis on the structural and functional properties of the pseudo-phosphorylated form of Tpm1.1 with the S283D substitution. We found that in acidosis, the interaction of the N- and C-ends of the S283D Tpm molecules decreases, whereas that of WT Tpm does not change. The pH lowering increased thermostability of the complex of F-actin with S283D Tpm to a greater extent than with WT Tpm. Using an in vitro motility assay with NEM- modified myosin as a load, we assessed the effect of the Tpm pseudo-phosphorylation on the force of the actin-myosin interaction. In acidosis, the force generated by myosin in the interaction with thin filaments containing S283D Tpm was higher than with those containing WT Tpm. Also, the pseudo-phosphorylation increased the myosin ability to resist a load. We conclude that ischemia changes the effect of the phosphorylated Tpm on the contractile function of the myocardium.




Similar content being viewed by others
References
Avner BS, Shioura KM, Scruggs SB et al (2012) Myocardial infarction in mice alters sarcomeric function via post-translational protein modification. Mol Cell Biochem 363(1–2):203–215. https://doi.org/10.1007/s11010-011-1172-z
Debold EP, Beck SE, Warshaw DM (2008) Effect of low pH on single skeletal muscle myosin mechanics and kinetics. Am J Physiol Cell Physiol 295(1):C173–C179. https://doi.org/10.1152/ajpcell.00172.2008
Fabiato A, Fabiato F (1978) Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol 276:233–255. https://doi.org/10.1113/jphysiol.1978.sp012231
Ford KL, Moorhouse EL, Bortolozzi M, Richards MA, Swietach P, Vaughan-Jones RD (2017) Regional acidosis locally inhibits but remotely stimulates Ca2+ waves in ventricular myocytes. Cardiovasc Res 113(8):984–995. https://doi.org/10.1093/cvr/cvx033
Fujita H, Sasaki D, Ishiwata S, Kawai M (2002) Elementary steps of the cross-bridge cycle in bovine myocardium with and without regulatory proteins. Biophys J 82:915–928. https://doi.org/10.1016/S0006-3495(02)75453-2
Garciarena CD, Ma YL, Swietach P, Huc L, Vaughan-Jones RD (2013) Sarcolemmal localisation of Na+/H+ exchange and Na+-HCO3- co-transport influences the spatial regulation of intracellular pH in rat ventricular myocytes. J Physiol 591(9):2287–2306. https://doi.org/10.1113/jphysiol.2012.249664
Gordon AM, Chen Y, Liang B, LaMadrid M, Luo Z, Chase PB (1998) Skeletal muscle regulatory proteins enhance F-actin in vitro motility. Adv Exp Med Biol 453:187–196
Gulati J, Babu A (1989) Effect of acidosis on Ca2+ sensitivity of skinned cardiac muscle with troponin C exchange Implications for myocardial ischemia. FEBS Lett 245(1–2):279–282. https://doi.org/10.1016/0014-5793(89)80237-6
Haeberle JR, Trybus KM, Hemric ME, Warshaw DM (1992) The effects of smooth muscle caldesmon on actin filament motility. J Biol Chem 267(32):23001–23006
Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH (1992) Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol 262(2 Pt 1):C348–C357. https://doi.org/10.1152/ajpcell.1992.262.2.C348
Heeley DH (1994) Investigation of the effects of phosphorylation of rabbit striated muscle alpha alpha-tropomyosin and rabbit skeletal muscle troponin-T. Eur J Biochem 221:129–137
Heeley DH, Moir AJ, Perry SV (1982) Phosphorylation of tropomyosin during development in mammalian striated muscle. FEBS Lett 146(1):115–118. https://doi.org/10.1016/0014-5793(82)80716-3
Heeley DH, Watson MH, Mak AS, Dubord P, Smillie LB (1989) Effect of phosphorylation on the interaction and functional properties of rabbit striated muscle alpha alpha-tropomyosin. J Biol Chem 264(5):2424–30
Homsher E, Nili M, Chen IY, Tobacman LS (2003) Regulatory proteins alter nucleotide binding to acto-myosin of sliding filaments in motility assays. Biophys J 85(2):1046–1052. https://doi.org/10.1016/S0006-3495(03)74543-3
Kohmoto O, Spitzer KW, Movsesian MA, Barry WH (1990) Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res 66(3):622–632. https://doi.org/10.1161/01.res.66.3.622
Kopylova GV, Shchepkin DV, Nabiev SR, Matyushenko AM, Koubassova NA, Levitsky DI, Bershitsky SY (2019) Cardiomyopathy-associated mutations in tropomyosin differently affect actin–myosin interaction at single-molecule and ensemble levels. J Muscle Res Cell Motil 40(3–4):299–308
Lehman W, Medlock G, Li XE et al (2015) Phosphorylation of Ser283 enhances the stiffness of the tropomyosin head-to-tail overlap domain. Arch Biochem Biophys 571:10–15. https://doi.org/10.1016/j.abb.2015.02.026
Lu X, Heeley DH, Smillie LB, Kawai M (2010) The role of tropomyosin isoforms and phosphorylation in force generation in thin-filament reconstituted bovine cardiac muscle fibres. J Muscle Res Cell Motil 31(2):93–109. https://doi.org/10.1007/s10974-010-9213-x
Mak A, Smillie LB, Barany M (1978) Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci USA 75(8):3588–3592. https://doi.org/10.1073/pnas.75.8.3588
Margossian SS, Lowey S (1982) Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol 85 Pt B:55‐71. doi:https://doi.org/10.1016/0076-6879(82)85009-x
Mashanov GI, Molloy JE (2007) Automatic detection of single fluorophores in live cells. Biophys J 92(6):2199–2211. https://doi.org/10.1529/biophysj.106.081117
Matyushenko AM, Artemova NV, Sluchanko NN, Levitsky DI (2015) Effects of two stabilizing substitutions, D137L and G126R, in the middle part of α-tropomyosin on the domain structure of its molecule. Biophys Chem 196:77–85. https://doi.org/10.1016/j.bpc.2014.10.001
Matyushenko AM, Shchepkin DV, Kopylova GV et al (2017) structural and functional effects of cardiomyopathy-causing mutations in the troponin T-binding region of cardiac tropomyosin. Biochemistry 56(1):250–259. https://doi.org/10.1021/acs.biochem.6b00994
Matyushenko AM, Kleymenov SY, Susorov DS, Levitsky DI (2018) Thermal unfolding of homodimers and heterodimers of different skeletal-muscle isoforms of tropomyosin. Biophys Chem 243:1–7. https://doi.org/10.1016/j.bpc.2018.09.002
McKillop DFA, Geeves MA (1993) Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65:693–701. https://doi.org/10.1016/S0006-3495(93)81110-X
Monteiro PB, Lataro RC, Ferro JA, Reinach Fde C (1994) Functional alpha-tropomyosin produced in Escherichia coli. A dipeptide extension can substitute the amino-terminal acetyl group. J Biol Chem 269(14):10461–10466
Pardee JD, Spudich JA (1982) Purification of muscle actin. Methods Enzymol 85 Pt B:164‐181. doi:https://doi.org/10.1016/0076-6879(82)85020-9
Perry SV (2001) Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil 22:5–49
Potter JD (1982) Preparation of troponin and its subunits. Methods Enzymol 85 Pt B:241‐263. doi:https://doi.org/10.1016/0076-6879(82)85024-6
Rajan S, Jagatheesan G, Petrashevskaya N, Biesiadecki BJ, Warren CM, Riddle T, Liggett S, Wolska BM, Solaro RJ, Wieczorek DF (2019) Tropomyosin pseudo-phosphorylation results in dilated cardiomyopathy. J Biol Chem 294(8):2913–2923. https://doi.org/10.1074/jbc.RA118.004879
Rao VS, La Bonte LR, Xu Y, Yang Z, French BA, Guilford WH (2007) Alterations to myofibrillar protein function in nonischemic regions of the heart early after myocardial infarction. Am J Physiol Heart Circ Physiol 293(1):654–659. https://doi.org/10.1152/ajpheart.01314.2006
Rao VS, Marongelli EN, Guilford WH (2009) Phosphorylation of tropomyosin extends cooperative binding of myosin beyond a single regulatory unit. Cell Motil Cytoskeleton 66(1):10–23. https://doi.org/10.1002/cm.20321
Reiser PJ, Kline WO (1998) Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol 274(3):1048–1053. https://doi.org/10.1152/ajpheart.1998.274.3.H1048
Sano K, Maeda K, Oda T, Maéda Y (2000) The effect of single residue substitutions of serine-283 on the strength of head-to-tail interaction and actin binding properties of rabbit skeletal muscle alpha-tropomyosin. J Biochem 127(6):1095–1102. https://doi.org/10.1093/oxfordjournals.jbchem.a022703
Sata M, Sugiura S, Yamashita H, Fujita H, Momomura S, Serizawa T (1995) MCI-154 increases Ca2+ sensitivity of reconstituted thin filament. A study using a novel in vitro motility assay technique. Circ Res 76(4):626–633. https://doi.org/10.1161/01.res.76.4.626
Schulz EM, Wieczorek DF (2013) Tropomyosin de-phosphorylation in the heart: what are the consequences? J Muscle Res Cell Motil. 34(3–4):239–46. https://doi.org/10.1007/s10974-013-9348-7
Schulz EM, Correll RN, Sheikh HN, Lofrano-Alves MS, Engel PL, Newman G, Schultz Jel J, Molkentin JD, Wolska BM, Solaro RJ, Wieczorek DF (2012) Tropomyosin dephosphorylation results in compensated cardiac hypertrophy. J Biol Chem 287(53):44478–89. https://doi.org/10.1074/jbc.M112.402040
Schulz EM, Wilder T, Chowdhury SA, Sheikh HN, Wolska BM, Solaro RJ, Wieczorek DF (2013) Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem 288(40):28925–28935. https://doi.org/10.1074/jbc.M113.466466
Shchepkin DV, Nabiev SR, Kopylova GV, Matyushenko AM, Levitsky DI, Bershitsky SY, Tsaturyan AK (2017) Cooperativity of myosin interaction with thin filaments is enhanced by stabilizing substitutions in tropomyosin. J Muscle Res Cell Motil 38(2):183–191
Solaro RJ (2011) Modulation of cardiac myofilament activity by protein phosphorylation. In: Page E, Fozzard HA, Solaro RJ (Eds) Handbook of physiology: section 2. The cardiovascular system. Oxford University Press, New York, NY, pp 264–300
Swietach P, Spitzer KW, Vaughan-Jones RD (2015) Na+ ions as spatial intracellular messengers for co-ordinating Ca2+ signals during pH heterogeneity in cardiomyocytes. Cardiovasc Res 105(2):171–181. https://doi.org/10.1093/cvr/cvu251
VanBuren P, Harris DE, Alpert NR, Warshaw DM (1995) Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ Res 77(2):439–44. https://doi.org/10.1161/01.res.77.2.439 (PMID: 7614728)
VanBuren P, Palmiter KA, Warshaw DM (1999) Tropomyosin directly modulates actomyosin mechanical performance at the level of a single actin filament. Proc Natl Acad Sci USA 96(22):12488–12493. https://doi.org/10.1073/pnas.96.22.12488
Warren CM, Arteaga GM, Rajan S, Ahmed RP, Wieczorek DF, Solaro RJ (2008) Use of 2-D DIGE analysis reveals altered phosphorylation in a tropomyosin mutant (Glu54Lys) linked to dilated cardiomyopathy. Proteomics 8(1):100–105. https://doi.org/10.1002/pmic.200700772
Woodward M, Previs MJ, Mader TJ, Debold EP (2015) Modifications of myofilament protein phosphorylation and function in response to cardiac arrest induced in a swine model. Front Physiol 6:199. https://doi.org/10.3389/fphys.2015.00199.eCollection2015
Acknowledgements
The authors thank Dr. Vera Borzova for assistance in the viscosity measurements and A. Kochurova for assistance in an in vitro motility assay experiments.
Funding
This work was funded by the Russian Foundation for Basic Research Grants 17-00-00065 (D.L.), 17-00-00070 (S.B.), 20-04-00130 (S.B.), and 18-34-20085 (D.S.); and State Program AAAA-A19-119010590010-3 (D.L.), and AAAA-A18-118020590135-3 (S.B.). This work was performed using the equipment of the Shared Research Center of Scientific Equipment of IIP UrB RAS. DSC measurements were carried out on the equipment of the Shared-Access Equipment Centre «Industrial Biotechnology» of Federal Research Center «Fundamentals of Biotechnology» of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kopylova, G.V., Matyushenko, A.M., Berg, V.Y. et al. Acidosis modifies effects of phosphorylated tropomyosin on the actin-myosin interaction in the myocardium. J Muscle Res Cell Motil 42, 343–353 (2021). https://doi.org/10.1007/s10974-020-09593-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-020-09593-4