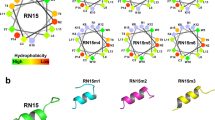
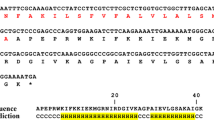
Abstract
Drug-resistant infectious diseases have increased in recent years. Accordingly, plenty of researches are exploring novel approaches to overcome this problem. In this era, antimicrobial peptides have been identified as potential antibacterial agents. The Modified CM11 (mCM11) was designed with the C-terminal amidation and substitution of lysine with arginine. The designed peptide was synthesized by the solid-phase method and Rink amide p-methyl-benzhydryl amine resin. The synthesized peptide was evaluated using Mass Spectrometry (MS), High-Performance Liquid Chromatography (HPLC), and Circular Dichroism (CD). Finally, the antibacterial, cytotoxic, and apoptotic effect of the mCM11 peptide was investigated. The new peptide indicated a beta-sheet structure with a molecular weight of 1527.50 D and purity of 96%. The peptide exerted a potent antimicrobial activity against gram-negative and positive bacteria. The minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) ranged from 16 to 64 µg/ml, and 16 to 128 µg/ml, respectively. The IC50 of mCM11 was 16 µg/ml and its cytotoxicity in SH-SY5Y cell line revealed a dose-dependent manner. Also, apoptosis analysis of eukaryotic cells revealed a decline in late apoptosis and necrosis in comparison with untreated cells. The mCM11 indicated a considerable antibacterial effect against a wide range of pathogenic bacterial strains. Further, it did not represent any late apoptotic and necrosis impact on the eukaryotic cell line. All of these findings may confirm the potential role of this new peptide as an effective therapeutic agent.






Similar content being viewed by others
References
Andreu D et al (1992) Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett 296(2):190–194
Beychok S (1966) Circular dichroism of biological macromolecules. Science 154(3754):1288–1299
Borro BC, Nordstrom R, Malmsten M (2020) Microgels and hydrogels as delivery systems for antimicrobial peptides. Colloids Surf B Biointerfaces 187:110835
Chung CR et al (2020) Characterization and Identification of Natural Antimicrobial Peptides on Different Organisms. Int J Mol Sci 21(3):986
Diaz-Achirica P et al (1998) The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1–8)M(1–18), a synthetic cecropin A-melittin hybrid peptide. Biochem J 330(Pt 1):453–460
Efimova SS, Schagina LV, Ostroumova OS (2014) Channel-forming activity of cecropins in lipid bilayers: effect of agents modifying the membrane dipole potential. Langmuir 30(26):7884–7892
Eloff JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64(8):711–713
Ferre R et al (2006) Inhibition of Plant-Pathogenic Bacteria by Short Synthetic Cecropin A-Melittin Hybrid Peptides. Appl Environ Microbiol 72(5):3302–3308
Gajski G, Garaj-Vrhovac V (2011) Bee venom induced cytogenetic damage and decreased cell viability in human white blood cells after treatment in vitro: a multi-biomarker approach. Environ Toxicol Pharmacol 32(2):201–211
Gajski G et al (2016) Melittin induced cytogenetic damage, oxidative stress and changes in gene expression in human peripheral blood lymphocytes. Toxicon 110:56–67
Gasteiger E et al (2005) Protein Identification and Analysis Tools on the ExPASy Server. In: Walker JM (ed) The Proteomics Protocols Handbook. Humana Press, Totowa, NJ, pp 571–607
Gautier R et al (2008) HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24(18):2101–2102
Humphries RM et al (2018) CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J Clin Microbiol 56(4):e01934-e2017
Jamsa A et al (2004) The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem Biophys Res Commun 319(3):993–1000
Jiang Z et al (2008) Effects of net charge and the number of positively charged residues on the biological activity of amphipathic alpha-helical cationic antimicrobial peptides. Biopolymers 90(3):369–383
Jiang X et al (2019) Design and activity study of a melittin-thanatin hybrid peptide. AMB Express 9(1):14–14
Juvvadi P et al (1996) Hydrophobic Effects on Antibacterial and Channel-forming Properties of Cecropin A-Melittin Hybrids. J Pept Sci 2(4):223–232
Karimi Tanha S, Pirestani M, Sadraei J (2019) Effect of Cecropin–melittin Chimeric Peptide on Trophozoite of Giardia lamblia In Vitro. Journal of Mazandaran University of Medical Sciences 29(177):42–55
Koriyama Y et al (2015) Glyceraldehyde caused Alzheimer’s disease-like alterations in diagnostic marker levels in SH-SY5Y human neuroblastoma cells. Sci Rep 5:13313
Lewies A, Du Plessis LH, Wentzel JF (2019) Antimicrobial Peptides: the Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicro Prot 11(2):370–381
Mackenzie D (2016) Antibiotic resistance hits crisis point. New Scientist 232(3104):32
Moghaddam MM et al (2012) Comparison of in vitro antibacterial activities of two cationic peptides CM15 and CM11 against five pathogenic bacteria: Pseudomonas aeruginosa, Staphylococcus aureus, Vibrio cholerae, Acinetobacter baumannii, and Escherichia coli. Probiotics Antimicrob Proteins 4(2):133–139
Moghaddam MM et al (2014) Investigation of the antibacterial activity of a short cationic peptide against multidrug-resistant Klebsiella pneumoniae and Salmonella typhimurium strains and its cytotoxicity on eukaryotic cells. World J Microbiol Biotechnol 30(5):1533–1540
N, N., et al (2014) Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujevac J Sci 36:129–136
Nuti R et al (2017) Antimicrobial Peptides: A Promising Therapeutic Strategy in Tackling Antimicrobial Resistance. Probiotics Antimicrob Proteins 24(38):4303–4314
Pizzolato-Cezar LR, Okuda-Shinagawa NM, Machini MT (2019) Combinatory Therapy Antimicrobial Peptide-Antibiotic to Minimize the Ongoing Rise of Resistance. Front Microbiol 10:1703
Polanco C et al (2012) Characterization of Selective Antibacterial Peptides by Polarity Index. International Journal of Peptides 2012:11
Rolain JM, Canton R, Cornaglia G (2012) Emergence of antibiotic resistance: need for a new paradigm. Clin Microbiol Infect 18(7):615–616
Rossolini GM et al (2014) Update on the antibiotic resistance crisis. Curr Opin Pharmacol 18:56–60
Saugar JM et al (2006) Activity of Cecropin A-Melittin Hybrid Peptides against Colistin-Resistant Clinical Strains of <em>Acinetobacter baumannii</em>: Molecular Basis for the Differential Mechanisms of Action. Antimicrob Agents Chemother 50(4):1251–1256
Sierra JM et al (2017) An overview of antimicrobial peptides and the latest advances in their development. Expert Opin Biol Ther 17(6):663–676
Steiner H (1982) Secondary structure of the cecropins: antibacterial peptides from the moth Hyalophora cecropia. FEBS Lett 137(2):283–287
Stockert JC et al (2018) Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem 120(3):159–167
Tassi M et al (2018) Advances in native high-performance liquid chromatography and intact mass spectrometry for the characterization of biopharmaceutical products. J Sep Sci 41:125–144
Terwilliger TC, Weissman L, Eisenberg D (1982) The structure of melittin in the form I crystals and its implication for melittin’s lytic and surface activities. Biophys J 37(1):353–361
Wade D et al (1992) Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res 40(5):429–436
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44(D1):D1087–D1093
Wei X-B et al (2016) Novel Hybrid Peptide Cecropin A (1–8)-LL37 (17–30) with Potential Antibacterial Activity. Int J Mol Sci 17(7):983
Wlodkowic D, Skommer J, Darzynkiewicz Z (2009) Flow cytometry-based apoptosis detection. Methods Mol Biol 559:19–32
Wu X et al (2016) Characterization of antimicrobial activity against Listeria and cytotoxicity of native melittin and its mutant variants. Colloids Surf B Biointerfaces 143:194–205
Yi HY et al (2014) Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol 98(13):5807–5822
Acknowledgments
This study was conducted on the scientific and spiritual support of the Islamic Azad University of Qom. The authors would also like to thank Mr. and Mrs. Mohammad Eshtiaghi, Mansoureh Algharnia, Samaneh Banaeian Kalaat, Zahra Karimi, Fahimeh Afzali, and all professors and students who helped with the projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
"Qom branch of Islamic Azad University" did not play any decision-making role in the study analysis or writing of the manuscript. All authors declare no Potential Conflicts of Interest.
Ethical Approval
As the present study involved no experimental with animals or humans, hence there was no need for approval by the Ethics Committee of Islamic Azad University, Qom branch.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eshtiaghi, S., Nazari, R. & Fasihi-Ramandi, M. In-Silico and In-Vitro Evaluation of Antibacterial, Cytotoxic, and Apoptotic Activity and Structure of Modified CM11 Peptide. Int J Pept Res Ther 27, 1069–1078 (2021). https://doi.org/10.1007/s10989-020-10151-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-020-10151-2