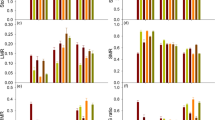
Abstract
Phragmites australis is a cosmopolitan plant species with high intraspecific diversity and phenotypic plasticity. Due to its variability and large ecological niche breadth, subgroups of P. australis have become invasive in North America, and this invasion has been recognized late. While this cryptic invasion on the American continent has received much attention, little is known about the potential invasiveness of other subgroups, especially within Asian/Australian P. australis. We therefore compared the performance of three subgroups within the Asian/Australian group: a freshwater (CN) and an estuarine (YRD) subgroup collected in China and a genetically closely related subgroup collected from Australia (FEAU), grown in two common gardens in China. Our results showed that the FEAU subgroup had no strong invasive potential, as its total biomass, height, shoot number, specific leaf area, and stomatal conductance were lower than that of the two native subgroups. All three subgroups responded similarly with most traits to the different climates of the gardens, albeit with different response strength, expressed as phenotypic plasticity indices. The potential cryptic invasion risk of the FEAU subgroup in China seems to be low, since its functional traits showed low competitiveness and most traits with the lowest plasticity occurred in FEAU. However, caution is still advised, because other invasive mechanisms, such as enemy release or the performance under extreme environmental conditions were not tested in our study.





Similar content being viewed by others
References
Abadia, J., 1992. Leaf responses to Fe deficiency: a review. Journal of Plant Nutrition 15(10): 1699–1713.
Abramoff, M. D., P. J. Magalhaes & S. J. Ram, 2004. Image processing with ImageJ. Biophotonics International 11: 36–42.
Albert, A., J. Brisson, F. Belzile, J. Turgeon & C. Lavoie, 2015. Strategies for a successful plant invasion: the reproduction of Phragmites australis in north-eastern North America. Journal of Ecology 103(6): 1861–1870.
Arve, L. E. & S. Torre, 2015. Ethylene is involved in high air humidity promoted stomatal opening of tomato (Lycopersicon esculentum) leaves. Functional Plant Biology 42(4): 376–386.
Aspinwall, M. J., D. B. Lowry, S. H. Taylor, T. E. Juenger, C. V. Hawkes, M. V. Johnson, J. R. Kiniry & P. A. Fay, 2013. Genotypic variation in traits linked to climate and aboveground productivity in a widespread C4 grass: evidence for a functional trait syndrome. New Phytologist 199(4): 966–980.
Banta, J. A., I. M. Ehrenreich, S. Gerard, L. Chou, A. Wilczek, J. Schmitt, P. X. Kover & M. D. Purugganan, 2012. Climate envelope modelling reveals intraspecific relationships among flowering phenology, niche breadth and potential range size in Arabidopsis thaliana. Ecology Letters 15(8): 769–777.
Bastlová, D., H. Čížková, M. Bastl & J. Květ, 2004. Growth of Lythrum salicaria and Phragmites australis plants originating from a wide geographical area: response to nutrient and water supply. Global Ecology and Biogeography 13(3): 259–271.
Bastlová, D., M. Bastl, H. Čížková & J. Květ, 2006. Plasticity of Lythrum salicaria and Phragmites australis growth characteristics across a European geographical gradient. Hydrobiologia 570(1): 237–242.
Baythavong, B. S. & M. L. Stanton, 2010. Characterizing selection on phenotypic plasticity in response to natural environmental heterogeneity. Evolution; International Journal of Organic Evolution 64(10): 2904–2920.
Bespalaya, Y. V., I. N. Bolotov, O. V. Aksenova, M. Y. Gofarov, A. V. Kondakov, I. V. Vikhrev & M. V. Vinarski, 2018. DNA barcoding reveals invasion of two cryptic Sinanodonta mussel species (Bivalvia: Unionidae) into the largest Siberian river. Limnologica 69: 94–102.
Bhattarai, G. P., L. A. Meyerson, J. Anderson, D. Cummings, W.J. Allen & J.T. Cronin, 2017. Biogeography of a plant invasion: genetic variation and plasticity in latitudinal clines for traits related to herbivory. Ecological Monographs 87(1): 57–75.
Bradshaw, A. D., 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115–155.
Brand, J. J. & D. W. Becker, 1984. Evidence for direct roles of calcium in photosynthesis. Journal of Bioenergetics and Biomembranes 16: 239–249.
Brix, H., S. Ye, E. A. Laws, D. Sun, G. Li, X. Ding, H. Yuan, G. Zhao, J. Wang & S. Pei, 2014. Large-scale management of common reed, Phragmites australis, for paper production: a case study from the Liaohe Delta, China. Ecological Engineering 73: 760–769.
Byrne, M. P. & P. A. O’Gorman, 2018. Trends in continental temperature and humidity directly linked to ocean warming. Proceedings of the National Academy of Sciences of the United States of America 115(19): 4863–4868.
Caplan, J. S., R. N. Hager, J. P. Megonigal & T. J. Mozdzer, 2015. Global change accelerates carbon assimilation by a wetland ecosystem engineer. Environmental Research Letters 10: 1–12.
Chambers, R. M., L. A. Meyerson & K. Saltonstall, 1999. Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany 64(3): 261–273.
Chambers, R. M., D. T. Osgood, D. J. Bart & F. Montalto, 2003. Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity, sulfide, and hydrology. Estuaries 26(2): 398–406.
Choi, W. & K. Y. Kim, 2018. Physical mechanism of spring and early summer drought over North America associated with the boreal warming. Scientific Reports 8(1): 7533.
Clements, D. R. & A. Ditommaso, 2011. Climate change and weed adaptation: can evolution of invasive plants lead to greater range expansion than forecasted? Weed Research 51(3): 227–240.
Clevering, O. A., 1998. An investigation into the effects of nitrogen on growth and morphology of stable and die-back populations of Phragmites australis. Aquatic Botany 60(1): 11–25.
Cornelissen, J. H. C., S. Lavorel, E. Garnier, S. Diaz, N. Buchmann, D. E. Gurvich, P. B. Reich, H. ter Steege, H. D. Morgan, M. G. van der Heijden, J. G. Pausas & H. Poorter, 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380.
Cramer, M. D., H.-J. Hawkins & G. A. Verboom, 2009. The importance of nutritional regulation of plant water flux. Oecologia 161: 15–24.
Cronin, J. T., G. P. Bhattarai, W. J. Allen & L. A. Meyerson, 2015. Biogeography of a plant invasion: plant–herbivore interactions. Ecology 96(4): 1115–1127.
Cronin, J. T., E. Kiviat, L. A. Meyerson, G. P. Bhattarai & W. J. Allen, 2016. Biological control of invasive Phragmites australis will be detrimental to native P. australis. Biological Invasions 18(9): 2749–2752.
Deng, X., W.-H. Ye, H.-L. Feng, Q.-H. Yang, H.-L. Cao, K.-Y. Xu & Y. Zhang, 2004. Gas Exchange Characteristics of the Invasive Species Mikania Micrantha and its Indigenous Congener M. Cordata (Asteraceae) in South China. Botanical Bulletin of Academia Sinica 45: 213–220.
Duan, X.-N., X.-K. Wang, Z.-Y. Ouyang, H. Miao & R. Guo, 2004. The biomass of Phragmites australis and its influencing factors in Wuliangsuhai. Acta Phytoecologica Sinica 28: 246–251. [in Chinese].
Eller, F. & H. Brix, 2012. Different genotypes of Phragmites australis show distinct phenotypic plasticity in response to nutrient availability and temperature. Aquatic Botany 103: 89–97.
Eller, F., H. Skalova, J. S. Caplan, G. P. Bhattarai, M. K. Burger, J. T. Cronin, W. Y. Guo, X. Guo, E. L. Hazelton, K. M. Kettenring, C. Lambertini, M. K. Mccormick, L. A. Meyerson, T. J. Mozdzer, P. Pyšek, B. K. Sorrell, D. F. Whigham & H. Brix, 2017. Cosmopolitan species as ecophysiological models for responses to global change: the common reed Phragmites australis. Frontiers in Plant Science 8: 1833.
Frevola, D. M. & S. M. Hovick, 2019. The independent effects of nutrient enrichment and pulsed nutrient delivery on a common wetland invader and its native conspecific. Oecologia 191(2): 447–460.
Geller, J. B., J. A. Darling & J. T. Carlton, 2010. Genetic perspectives on marine biological invasions. Annual review of marine science 2: 367–393.
Gerlach, J. D., B. S. Bushman, J. K. Mckay & H. Meimberg, 2009. Taxonomie confusion permits the unchecked invasion of vernal pools in California by low mannagrass vena deelinafa). Invasive Plant Science & Management 2(1): 92–97.
Ghalambor, C. K., J. K. McKay, S. P. Carroll & D. N. Reznick, 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21(3): 394–407.
Gitelson, A. A., C. Buschmann & H. K. Lichtenthaler, 1999. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sensing of Environment 69(3): 296–302.
Guo, W. Y., C. Lambertini, X. Z. Li, L. A. Meyerson & H. Brix, 2013. Invasion of Old World Phragmites australis in the New World: precipitation and temperature patterns combined with human influences redesign the invasive niche. Global Change Biology 19(11): 3406–3422.
Hansen, D. L., C. Lambertini, A. Jampeetong & H. Brix, 2007. Clone-specific differences in Phragmites australis: effects of ploidy level and geographic origin. Aquatic Botany 86: 269–279.
Henn, J. J., S. Yelenik & E. I. Damschen, 2019. Environmental gradients influence differences in leaf functional traits between native and nonnative plants. Oecologia 191: 397–409.
Hetherington, A. M. & F. I. Woodward, 2003. The role of stomata in sensing and driving environmental change. Nature 424(6951): 901–908.
Hurry, C. R., E. A. James & R.M. Thompson, 2013. Connectivity, genetic structure and stress response of Phragmites australis: Issues for restoration in a salinising wetland system. Aquatic Botany 104: 138–146.
Jaric, I., T. Heger, F. C. Monzon, J. M. Jeschke, I. Kowarik, K. R. McConkey, P. Pyšek, A. Sagouis & F. Essl, 2019. Crypticity in biological invasions. Trends in Ecology and Evolution 34(4): 291–302.
Kalaji, H. M., A. Oukarroum, V. Alexandrov, M. Kouzmanova, M. Brestic, M. Zivcak, I. A. Samborska, M. D. Cetner, S. I. Allakhverdiev & V. Goltsev, 2014. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiology and Biochemistry 81: 16–25.
Karunaratne, S., T. Asaeda & K. Yutani, 2003. Growth performance of Phragmites australis in Japan: influence of geographic gradient. Environmental and Experimental Botany 50(1): 51–66.
Keane, R. M. & M. J. Crawley, 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution 17(4): 164–170.
Kulmatiski, A., K. H. Beard, L. A. Meyerson, J. R. Gibson & K. E. Mock, 2010. Nonnative Phragmites australis invasion into Utah wetlands. Western North American Naturalist 70(4): 541–552.
Kupper, P., G. Rohula, L. Inno, I. Ostonen, A. Sellin & A. Sõber, 2017. Impact of high daytime air humidity on nutrient uptake and night-time water flux in silver birch, a boreal forest tree species. Regional Environmental Change 17(7): 2149–2157.
Laing, W., D. Greer, O. Sun, P. Beets, A. Lowe & T. Payn, 2000. Physiological impacts of Mg deficiency in Pinus radiata: growth and photosynthesis. New Phytologist 146(1): 47–57.
Lamarque, L. J., C. J. Lortie, A. J. Porté & S. Delzon, 2015. Genetic differentiation and phenotypic plasticity in life-history traits between native and introduced populations of invasive maple trees. Biological Invasions 17(4): 1109–1122.
Lambertini, C., B. K. Sorrell, T. Riis, B. Olesen & H. Brix, 2012. Exploring the borders of European Phragmites within a cosmopolitan genus. AoB PLANTS. https://doi.org/10.1093/aobpla/pls020.
Lambertini, C., W. Y. Guo, S. Ye, F. Eller, X. Guo, X. Z. Li, B. K. Sorrell, M. Speranza & H. Brix, 2020. Phylogenetic diversity shapes salt tolerance in Phragmites australis estuarine populaions in East China. Scientific Reports 10: 17645.
Lavergne, S. & J. Molofsky, 2007. Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proceedings of the National Academy of Sciences of the United States of America 104(10): 3883–3888.
Leuschner, C., 2002. Air humidity as an ecological factor for woodland herbs: leaf water status, nutrient uptake, leaf anatomy, and productivity of eight species grown at low or high VPD levels. Flora 197(4): 262–274.
Marks, M., B. Lapin & J. Randall, 1994. Phragmites australis (P. communis): threats, management and monitoring. Natural Areas Journal 14(4): 285–294.
Marrone, F., S. Lo Brutto & M. Arculeo, 2011. Cryptic invasion in Southern Europe: the case of Ferrissia fragilis (Pulmonata: Ancylidae) Mediterranean populations. Biologia 66(3): 484–490.
Matzek, V., 2011. Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biological Invasions 13(12): 3005.
McAlpine, K. G., L. K. Jesson & D. S. Kubien, 2008. Photosynthesis and water-use efficiency: a comparison between invasive (exotic) and non-invasive (native) species. Austral Ecology 33(1): 10–19.
McDowell, S. C. L., 2002. Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). American Journal of Botany 89(9): 1431–1438.
Meadows, R. E. & K. Saltonstall, 2007. Distribution of native and non-native populations of Phragmites australis in oligohaline marshes of the Delmarva Peninsula and southern New Jersey. Journal of the Torrey Botanical Society 134(1): 99–107.
Messier, J., M. J. Lechowicz, B. J. McGill, C. Violle & B. J. Enquist, 2017. Interspecific integration of trait dimensions at local scales: the plant phenotype as an integrated network. Journal of Ecology 105(6): 1775–1790.
Meyerson, L. A., K. Saltonstall, L. Windham, E. Kiviat & S. Findlay, 2000. A comparison of Phragmites australisin freshwater and brackish marsh environments in North America. Wetlands Ecology and Management 8(2): 89–103.
Meyerson, L. A., K. Saltonstall, R. M. Chambers, B. R. Silliman, M. D. Bertness & D. Strong, 2009. Phragmites australis in Eastern North America: A Historical and Ecological Perspective. Univeristy of California Press, Berkeley.
Meyerson, L. A., C. Lambertini, M. K. Mccormick & D. F. Whigham, 2012. Hybridization of common reed in North America? The answer is blowing in the wind. AoB Plants 2012(1): pls022.
Meyerson, L. A., J. T. Cronin & P. Pyšek, 2016. Phragmites australis as a model organism for studying plant invasions. Biological Invasions 18(9): 2421–2431.
Minchinton, T. E., 2002. Precipitation during El Niño correlates with increasing spread of Phragmites australis in New England, USA, coastal marshes. Marine Ecology Progress 242(1): 305–309.
Monty, A., J. P. Bizoux, J. Escarré & G. Mahy, 2013. Rapid plant invasion in distinct climates involves different sources of phenotypic variation. PLoS ONE 8(1): e55627.
Mooney, H. A. & R. J. Hobbs, 2000. Invasive Species in a Changing World. Island Press, Washington.
Moroney, J. R., P. W. Rundel & V. L. Sork, 2013. Phenotypic plasticity and differentiation in fitness-related traits in invasive populations of the Mediterranean forb Centaurea melitensis (Asteraceae). American Journal of Botany 100(10): 2040–2051.
Morris, K., P. I. Boon, E. J. Raulings & S. D. White, 2008. Floristic shifts in wetlands: the effects of environmental variables on the interaction between Phragmites australis (Common Reed) and Melaleuca ericifolia (Swamp Paperbark). Marine and Freshwater Research 59(3): 187–204.
Mozdzer, T. J. & J. P. Megonigal, 2012. Jack-and-master trait responses to elevated CO2 and N: a comparison of native and introduced Phragmites australis. PLoS ONE 7(10): e42794.
Mozdzer, T. J., J. Brisson & E. L. G. Hazelton, 2013. Physiological ecology and functional traits of North American native and Eurasian introduced Phragmites australis lineages. AoB Plants 5: plt048.
Munns, R. & M. Tester, 2008. Mechanisms of salinity tolerance. Annual Review of Plant Biology 59: 651–681.
Nicotra, A. B., O. K. Atkin, S. P. Bonser, A. M. Davidson, E. J. Finnegan, U. Mathesius, P. Poot, M. D. Purugganan, C. L. Richards, F. Valladares & M. van Kleunen, 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15(12): 684–692.
Ocheltree, T. W., J. B. Nippert & P. V. V. Prasad, 2012. Changes in stomatal conductance along grass blades reflect changes in leaf structure. Plant, Cell and Environment 35(6): 1040–1049.
Osman, K. T., 2013. Plant nutrients and soil fertility management. Soils 2013: 129–159.
Packer, J. G., L. A. Meyerson, D. M. Richardson, G. Brundu, W. J. Allen, G. P. Bhattarai, H. Brix, S. Canavan, S. Castiglione, A. Cicatelli, J. Čuda, J. T. Cronin, F. Eller, F. Guarino, W.-H. Guo, W.-Y. Guo, X. Guo, J. L. Hierro, C. Lambertini, J. Liu, V. Lozano, T. J. Mozdzer, H. Skálová, D. Villarreal, R.-Q. Wang & P. Pyšek, 2017. Global networks for invasion science: benefits, challenges and guidelines. Biological Invasions 19(4): 1081–1096.
Pauls, S. U., C. Nowak, M. Balint & M. Pfenninger, 2013. The impact of global climate change on genetic diversity within populations and species. Molecular Ecology 22(4): 925–946.
Philipp, K. R. & R. T. Field, 2005. Phragmites australis expansion in Delaware Bay salt marshes. Ecological Engineering 25(3): 275–291.
Pimentel, D., R. Zuniga & D. Morrison, 2005. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics 52(3): 273–288.
Poorter, H., K. J. Niklas, P. B. Reich, J. Oleksyn, P. Poot & L. Mommer, 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist 193(1): 30–50.
Pyšek, P., H. Skálová, J. Čuda, W.-Y. Guo, J. Doležal, O. Kauzál, C. Lambertini, K. Pyšková, H. Brix & L. A. Meyerson, 2019. Physiology of a plant invasion: biomass production, growth and tissue chemistry of invasive and native Phragmites australis populations. Preslia 91: 51–75.
Pyšek, P., J. Čuda, P. Šmilauer, H. Skálová, Z. Chumová, C. Lambertini, M. Lučanová, H. Ryšavá, P. Trávníček, K. Šemberová & L. A. Meyerson, 2020. Competition among native and invasive Phragmites australis populations: an experimental test of the effects of invasion status, genome size, and ploidy level. Ecology and Evolution 10: 1106–1118.
Ren, L., X. Guo, S. Liu, T. Yu, W. Guo, R. Wang, S. Ye, C. Lambertini, H. Brix & F. Eller, 2020. Intraspecific variation in Phragmites australis: Clinal adaption of functional traits and phenotypic plasticity vary with latitude of origin. Journal of Ecology 108(6): 2531–2543.
Rice, D., J. Rooth & J. C. Stevenson, 2000. Colonization and expansion of Phragmites australis in upper Chesapeake Bay tidal marshes. Wetlands 20(2): 280–299.
Rüegg, S., U. Raeder, A. Melzer, G. Heubl & C. Bräuchler, 2017. Hybridisation and cryptic invasion in Najas marina L. (Hydrocharitaceae)? Hydrobiologia 784(1): 381–395.
Sala, O. E., F. S. Chapin 3rd, J. J. Armesto, E. Berlow, J. Bloomfield, R. Dirzo, E. Huber-Sanwald, L. F. Huenneke, R. B. Jackson, A. Kinzig, R. Leemans, D. M. Lodge, H. A. Mooney, M. Oesterheld, N. L. Poff, M. T. Sykes, B. H. Walker, M. Walker & D. H. Wall, 2000. Global biodiversity scenarios for the year 2100. Science 287(5459): 1770–1774.
Saltonstall, K., 2002. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences of the United States of America 99(4): 2445–2449.
Saltonstall, K., P. L. Peterson & R. J. Soreng, 2004. Recognition of Phragmites australis subsp. americanus (Poaceae: Arundinoideae) in North America: evidence from morphological and genetic analyses. SIDA 21(2): 683–692.
Silliman, B. R. & M. D. Bertness, 2004. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conservation Biology 18(5): 1424–1434.
Silva, L. C. R. & H. Lambers, 2020. Soil–plant–atmosphere interactions: structure, function, and predictive scaling for climate change mitigation. Plant and Soil. https://doi.org/10.1007/s11104-020-04427-1.
Tulbure, M. G. & C. A. Johnston, 2010. Environmental conditions promoting non-native Phragmites australis expansion in Great Lakes coastal wetlands. Wetlands 30(3): 577–587.
Tulbure, M. G., C. A. Johnston & D. L. Auger, 2007. Rapid invasion of a Great Lakes coastal wetland by non-native Phragmites australis and Typha. Journal of Great Lakes Research 33: 269–279.
Uddin, M. N., R. W. Robinson, A. Buultjens, M. A. Y. Al Harun & S. H. Shampa, 2017. Role of allelopathy of Phragmites australis in its invasion processes. Journal of Experimental Marine Biology and Ecology 486: 237–244.
Valladares, F., D. Sanchez-Gomez & M. A. Zavala, 2006. Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology 94(6):1103–1116.
Valladares, F., S. Matesanz, F. Guilhaumon, M. B. Araujo, L. Balaguer, M. Benito-Garzon, W. Cornwell, E. Gianoli, M. van Kleunen, D. E. Naya, A. B. Nicotra, H. Poorter & M. A. Zavala, 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecology Letters 17(11): 1351–1364.
van Kleunen, M., E. Weber & M. Fischer, 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13(2): 235–245.
Vasquez, E. A., E. P. Glenn, J. J. Brown, G. R. Guntenspergen & S. G. Nelson, 2005. Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Marine Ecology Progress Series 298(1): 1–8.
Violle, C., M. L. Navas, D. Vile, E. Kazakou, C. Fortunel, I. Hummel & E. Garnier, 2007. Let the concept of trait be functional! Oikos 116: 882–892.
Vitousek, P. M., C. M. D’Antonio, L. L. Loope & R. Westbrooks, 1996. Biological invasions as global environmental change. American Scientist 84(5): 468–478.
Walck, J. L., J. M. Baskin & C. C. Baskin, 2001. Why is Solidago shortii narrowly endemic and S. altissima geographically widespread? A comprehensive comparative study of biological traits. Journal of Biogeography 28(10): 1221–1237.
Wang, I. J., R. E. Glor & J. B. Losos, 2013. Quantifying the roles of ecology and geography in spatial genetic divergence. Ecology Letters 16(2): 175–182.
White, T. A., B. D. Campbell, P. D. Kemp & C. L. Hunt, 2001. Impacts of extreme climatic events on competition during grassland invasions. Global Change Biology 7(1): 1–13.
Yamori, W., K. Hikosaka & D. A. Way, 2014. Temperature response of photosynthesis in C3, C4, and CAM plants: temperature acclimation and temperature adaptation. Photosynthesis Research 119(1): 101–117.
Zheng, Y.-L., Y.-L. Feng, W.-X. Liu & Z.-Y. Liao, 2009. Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecology 203(2): 263–271.
Acknowledgements
This study was jointly funded by the National Key R&D Program of China (Grant No. 2016YFE0109600), Ministry of Land and Resources program: “Special foundation for scientific research on public causes” (Grant No. 201111023), National Natural Science Foundation of China (Grant Nos. 41240022, 40872167, 31970347 and 31770361), and China Geological Survey (Grant Nos. DD20189503 and GZH201200503). E. Jespersen was supported by the Sino-Danish Center for Education and Research (SDC). F. Eller was funded by a grant from the Carlsberg Foundation (Grant No. CF15-0330). This manuscript benefitted greatly from the constructive comments of editor and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Franziska Eller, Hans Brix, Brian K. Sorrell & Carlos A. Arias / Wetland ecosystems: functions and use in a changing climate
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, L., Jespersen, E., Ye, S. et al. Intraspecific differences of Asian/Australian Phragmites australis subgroups reveal no potentially invasive traits. Hydrobiologia 848, 3331–3351 (2021). https://doi.org/10.1007/s10750-020-04474-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04474-w