Abstract
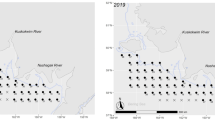
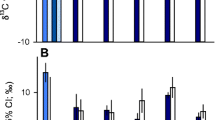
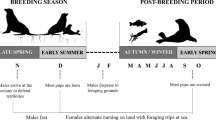
Differences in reproductive allocation of male and female fishes could lead to sexual divergence in nutritional requirements, trophic ecologies, and metabolisms that may be reflected in isotopic compositions. We compared muscle C:N ratio, δ13C, and δ15N between mature females and males for eight fish species from 21 North American populations. We also compared isotopic compositions between muscle and ova in a subset of females to determine if observed sex differences may be associated with fractionation during oogenesis. Muscle δ13C and δ15N did not vary greatly between males and females for most species but significant differences were observed in walleye (Sander vitreus) and burbot (Lota lota). Among-individual variation in isotopic composition, estimated as dispersion in δ13C–δ15N space, did not differ significantly between the sexes. Ova had significantly higher C:N ratio and lower δ13C than female muscle in all species with the greatest contrast between tissues seen in burbot. Ova had significantly higher δ15N than female muscle in most species with the greatest contrast between tissues in walleye. Sex-based divergence in isotopic composition of northern fishes is most evident in lean-fleshed piscivores, and may be related to selective mobilization of nutrients from soma to ovaries.



Similar content being viewed by others
Data availability
The datasets generated by and analysed for the current study are available from the corresponding author on reasonable request.
References
Al-Yousuf, M. H., M. S. El-Shahawi & S. M. Al-Ghais, 2000. Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Science of the Total Environment 256: 87–94.
Andersson, M. B., 1994. Sexual Selection. Princeton University Press, Princeton, New Jersey.
Barnes, C., C. J. Sweeting, S. Jennings, J. T. Barry & N. V. C. Polunin, 2007. Effect of temperature and ration size on carbon and nitrogen stable isotope trophic fractionation. Functional Ecology 21: 356–362.
Barnes, C., S. Jennings, N. V. C. Polunin & J. E. Lancaster, 2008. The importance of quantifying inherent variability when interpreting stable isotope field data. Oecologia 155: 227–235.
Batschelet, E., 1981. Circular Statistics in Biology. Academic Press, New York, NY.
Bearhop, S., R. A. Phillips, R. McGill, Y. Cherel, D. A. Dawson & J. P. Croxall, 2006. Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Marine Ecology Progress Series 311: 157–164.
Bilby, R. E., B. R. Fransen & P. A. Bisson, 1996. Incorporation of nitrogen and carbon from spawning coho salmon into the trophic system of small streams: evidence from stable isotopes. Canadian Journal of Fisheries and Aquatic Sciences 53: 164–173.
Boecklen, W. J., C. T. Yarnes, B. A. Cook & A. C. James, 2011. On the use of stable isotopes in trophic ecology. Annual Review of Ecology Evolution and Systematics 42: 411–440.
Bolnick, D. I., L. K. Snowberg, P. E. Hirsch, C. L. Lauber, R. Knight, J. G. Caporaso & R. Svanbäck, 2014. Individuals’ diet diversity influences gut microbial diversity in two freshwater fish (threespine stickleback and Eurasian perch). Ecology Letters 17: 979–987.
Borrell, A., A. Aguilar, M. Gazo, R. P. Kumarran & L. Cardona, 2011. Stable isotope profiles in whale shark (Rhincodon typus) suggest segregation and dissimilarities in the diet depending on sex and size. Environmental Biology of Fishes 92: 559–567.
Box, A., S. Deudero, A. Blanco, A. M. Grau & F. Riera, 2010. Differences in δ13C and δ15N stable isotopes in the pearly razorfish Xyrichtys novacula related to the sex, location and spawning period. Journal of Fish Biology 76: 2370–2381.
Bulté, G., M. A. Gravel & G. Blouin-Demers, 2008. Intersexual niche divergence in northern map turtles (Graptemys geographica): the roles of diet and habitat. Canadian Journal of Zoology 86: 1235–1243.
Burger, J., 2007. A framework and methods for incorporating gender-related issues in wildlife risk assessment: gender-related differences in metal levels and other contaminants as a case study. Environmental Research 104: 153–162.
Camilleri, C. & R. Shine, 1990. Sexual dimorphism and dietary divergence: differences in trophic morphology between male and female snakes. Copeia 1990: 649–658.
Casselman, S. J. & A. I. Schulte-Hostedde, 2004. Reproductive roles predict sexual dimorphism in internal and external morphology of lake whitefish, Coregonus clupeaformis. Ecology of Freshwater Fish 13: 217–222.
Catry, I., T. Catry, M. Alho, A. M. A. Franco & F. Moreira, 2016. Sexual and parent–offspring dietary segregation in a colonial raptor as revealed by stable isotopes. Journal of Zoology 299: 58–67.
Cherel, Y., C. Fontaine, G. D. Jackson, C. H. Jackson & P. Richard, 2009. Tissue, ontogenic and sex-related differences in δ13C and δ15N values of the oceanic squid Todarodes filippovae (Cephalopoda: Ommastrephidae). Marine Biology 156: 699–708.
Chikaraishi, Y., N. O. Ogawa, Y. Kashiyama, Y. Takano, H. Suga, A. Tomitani, H. Miyashita, H. Kitazato & N. Ohkouchi, 2009. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnology and Oceanography: Methods 7: 740–750.
Cott, P. A., T. A. Johnston & J. M. Gunn, 2013. Sexual dimorphism in an under-ice spawning fish: the burbot (Lota lota). Canadian Journal of Zoology 91: 732–740.
Darwin, C., 1874. The descent of man, and selection in relation to sex, 2nd ed. John Murray, London.
Dehnhard, N., C. C. Voigt, M. Poisbleau, L. Demongin & P. Quillfeldt, 2011. Stable isotopes in southern rockhopper penguins: foraging areas and sexual differences in the non-breeding period. Polar Biology 34: 1763–1773.
Focken, U. & K. Becker, 1998. Metabolic fractionation of stable carbon isotopes: implications of different proximate compositions for studies of the aquatic food webs using δ13C data. Oecologia 115: 337–343.
Forero, M. G., K. A. Hobson, G. R. Bortolotti, J. A. Donazar, M. Bertellotti & G. Blanco, 2002. Food resource utilisation by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Marine Ecology Progress Series 234: 289–299.
Forero, M. G., J. Gonzalez-Solis, K. A. Hobson, J. A. Donazar, M. Bertellotti, G. Blanco & G. R. Bortolotti, 2005. Stable isotopes reveal trophic segregation by sex and age in the southern giant petrel in two different food webs. Marine Ecology Progress Series 296: 107–113.
Frankel, N. S., H. B. Vander Zanden, K. J. Reich, K. L. Williams & K. A. Bjorndal, 2012. Mother−offspring stable isotope discrimination in loggerhead sea turtles Caretta caretta. Endangered Species Research 17: 133–138.
Fry, B., 2006. Stable Isotope Ecology. Springer, New York.
García-Tarrasón, M., J. Bécares, S. Bateman, J. M. Arcos, L. Jover & C. Sanpera, 2015. Sex-specific foraging behavior in response to fishing activities in a threatened seabird. Ecology and Evolution 5: 2348–2358.
Gaye-Siessegger, J., U. Focken, S. Muetzel, H. Abel & K. Becker, 2004. Feeding level and individual metabolic rate affect δ13C and δ15N values in carp: implications for food web studies. Oecologia 138: 175–183.
Hanisch, J. R., W. M. Tonn, C. A. Paszkowski & G. J. Scrimgeour, 2010. δ13C and δ15N signatures in muscle and fin tissues: nonlethal sampling methods for stable isotope analysis of salmonids. North American Journal of Fisheries Management 30: 1–11.
Henderson, R. J., J. R. Sargent & C. C. E. Hopkins, 1984. Changes in the content and fatty acid composition of lipid in an isolated population of the capelin Mallotus villosus during sexual maturation and spawning. Marine Biology 78: 255–263.
Henderson, B. A., N. Collins, G. E. Morgan & A. Vaillancourt, 2003. Sexual size dimorphism of walleye (Stizostedion vitreum vitreum). Canadian Journal of Fisheries and Aquatic Sciences 60: 1345–1352.
Houston, D. & R. Shine, 1993. Sexual dimorphism and niche divergence: feeding habits of the Arafura filesnake. Journal of Animal Ecology 62: 737–748.
Iwasaki, M. & R. Harada, 1985. Proximate and amino acid composition of the roe and muscle of selected marine species. Journal of Food Science 50: 1585–1587.
Jackson, A. L., R. Inger, A. C. Parnell & S. Bearhop, 2011. Comparing isotopic niche widths among and within communities: SIBER – Stable Isotope Bayesian Ellipses in R. Journal of Animal Ecology 80: 595–602.
Jardine, T. D., M. A. Gray, S. M. McWilliam & R. A. Cunjak, 2005. Stable isotope variability in tissues of temperate stream fishes. Transactions of the American Fisheries Society 134: 1103–1110.
Jardine, T. D., K. A. Kidd & A. T. Fisk, 2006. Applications, considerations, and sources of uncertainty when using stable isotope analysis in ecotoxicology. Environmental Science and Technology 40: 7501–7511.
Johnston, T. A., A. T. Fisk, D. M. Whittle & D. C. G. Muir, 2002. Variation in organochlorine bioaccumulation by a predatory fish; gender, geography, and data analysis methods. Environmental Science and Technology 36: 4238–4244.
Johnston, T. A., D. M.-M. Wong, M. D. Moles, M. D. Wiegand, J. M. Casselman & W. C. Leggett, 2012. Reproductive allocation in exploited lake whitefish (Coregonus clupeaformis) and walleye (Sander vitreus) populations. Fisheries Research 125–126: 225–234.
Johnston, T. A., M. C. Prévost, L. C. Haslam & P. A. Addison, 2016. Life history variation within and among naturalized rainbow trout populations of the Laurentian Great Lakes. Journal of Great Lakes Research 42: 861–870.
Johnston, T. A., A. D. Ehrman, J. J. Montgomery & H. K. Swanson, 2020a. Dietary and non-dietary contributions to among-individual variation in isotopic composition of lake trout. Ecological Indicators, accepted pending revision, 20 Nov 2020.
Johnston, T. A., M. D. Wiegand, R. L. Szmadyla, L. R. Porteous, J. M. Casselman & W. C. Leggett, 2020b. Sex-based differences in fatty acid composition of adult walleye. Ecology of Freshwater Fish 29: 654–664.
Karachle, P. K. & K. I. Stergiou, 2008. The effect of season and sex on trophic levels of marine fishes. Journal of Fish Biology 72: 1463–1487.
Kernaléguen, L., B. Cazelles, J. P. Y. Arnould, P. Richard, C. Guinet & Y. Cherel, 2012. Long-term species, sexual and individual variations in foraging strategies of fur seals revealed by stable isotopes in whiskers. PLoS ONE 7: e32916.
Layman, C. A., D. A. Arrington, C. G. Montana & D. M. Post, 2007. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88: 42–48.
Layman, C. A., M. S. Araujo, R. Boucek, C. M. Hammerschlag-Peyer, E. Harrison, Z. R. Jud, P. Matich, A. E. Rosenblatt, J. J. Vaudo, L. A. Yeager, D. M. Post & S. Bearhop, 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological Reviews 87: 545–562.
Lewis, R., T. C. O’Connell, M. Lewis, C. Carnpagna & A. R. Hoelzel, 2006. Sex-specific foraging strategies and resource partitioning in the southern elephant seal (Mirounga leonina). Proceedings of the Royal Society of London B 273: 2901–2907.
Logan, J. M., T. D. Jardine, T. J. Miller, S. E. Bunn, R. A. Cunjak & M. E. Lutcavage, 2008. Lipid corrections in carbon and nitrogen stable isotope analyses: comparison of chemical extraction and modelling methods. Journal of Animal Ecology 77: 838–846.
Madenjian, C. P., 2011. Sex effect on polychlorinated biphenyl concentrations in fish: a synthesis. Fish and Fisheries 12: 451–460.
Madenjian, C. P., G. E. Noguchi, R. C. Haas & K. S. Schrouder, 1998. Sexual difference in polychlorinated biphenyl accumulation rates of walleye (Stizostedion vitreum). Canadian Journal of Fisheries and Aquatic Sciences 55: 1085–1092.
Madenjian, C. P., M. A. Stapanian, P. A. Cott, R. R. Rediske & J. P. O’Keefe, 2014. Polychlorinated biphenyl concentrations of burbot Lota lota from Great Slave Lake are very low but vary by sex. Archives of Environmental Contamination and Toxicology 66: 529–537.
Mariano-Jelicich, R., F. Botto, P. Martinetto, O. Iribarne & M. Favero, 2008. Trophic segregation between sexes in the Black Skimmer revealed through the analysis of stable isotopes. Marine Biology 155: 443–450.
McMahon, K. W. & M. D. McCarthy, 2016. Embracing variability in amino acid δ15N fractionation: mechanisms, implications, and applications for trophic ecology. Ecosphere 7: e01511.
McMeans, B. C., J. A. Olin & G. W. Benz, 2009. Stable-isotope comparisons between embryos and mothers of a placentatrophic shark species. Journal of Fish Biology 75: 2464–2474.
Meik, J. M., K. Setser, E. Mociño-Deloya & A. M. Lawing, 2012. Sexual differences in head form and diet in a population of Mexican lance-headed rattlesnakes, Crotalus polystictus. Biological Journal of the Linnean Society 106: 633–640.
Moles, M. D., T. A. Johnston, B. W. Robinson, W. C. Leggett & J. M. Casselman, 2008. Is gonadal investment in walleye (Sander vitreus) dependent on body lipid reserves? A multipopulation comparative analysis. Canadian Journal of Fisheries and Aquatic Sciences 65: 600–614.
Morehouse, N. I., T. Nakazawa, C. M. Booher, P. D. Jeyasingh & M. D. Hall, 2010. Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119: 766–778.
Navarro, J., D. Oro, A. Bertolero, M. Genovart, A. Delgado & M. G. Forero, 2010. Age and sexual differences in the exploitation of two anthropogenic food resources for an opportunistic seabird. Marine Biology 157: 2453–2459.
Newsome, S. D., C. M. del Rio, S. Bearhop & D. L. Phillips, 2007. A niche for isotopic ecology. Frontiers in Ecology and the Environment 5: 429–436.
Nicoletto, P. F. & A. C. Hendricks, 1988. Sexual differences in accumulation of mercury in four species of centrarchid fishes. Canadian Journal of Zoology 66: 944–949.
Perry, G., 1996. The evolution of sexual dimorphism in the lizard Anolis polylepis (Iguania): evidence from intraspecific variation in foraging behavior and diet. Canadian Journal of Zoology 74: 1238–1245.
Peterson, B. J. & B. Fry, 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Pinnegar, J. K. & N. V. C. Polunin, 1999. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225–231.
Post, D. M., C. A. Layman, D. A. Arrington, G. Takimoto, J. Quattrochi & C. G. Montana, 2007. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152: 179–189.
Power, M., K. R. R. A. Guiguer & D. R. Barton, 2003. Effects of temperature on isotopic enrichment in Daphnia magna: implications for aquatic food-web studies. Rapid Communications in Mass Spectrometry 17: 1619–1625.
Pritchard, H., K. Langford & S. E. Mann, 2019. Methods for preparing calcified fish structures for age interpretation. Science and Research Branch Technical Manual TM-09. Ontario Ministry of Natural Resources and Forestry, Peterborough, ON.
Quillfeldt, P., R. A. R. McGill, J. F. Masello, F. Weiss, I. J. Strange, P. Brickle & R. W. Furness, 2008. Stable isotope analysis reveals sexual and environmental variability and individual consistency in foraging of thin-billed prions. Marine Ecology Progress Series 373: 137–148.
SAS Institute Inc, 2013. SAS OnlineDoc® Version 9.4. SAS Institute Inc, Cary, NC.
Shine, R., 1989. Ecological causes for the evolution of sexual dimorphism: a review of the evidence. Quarterly Review of Biology 64: 419–462.
Shine, R., P. S. Harlow, J. S. Keogh & Boeadi, 1998. The influence of sex and body size on food habits of a giant tropical snake, Python reticulatus. Functional Ecology 12: 248–258.
Sweeting, C. J., J. Barry, C. Barnes, N. V. C. Polunin & S. Jennings, 2007. Effects of body size and environment on diet-tissue δ15N fractionation in fishes. Journal of Experimental Marine Biology and Ecology 340: 1–10.
Trueman, C. N., R. A. R. McGill & P. H. Guyard, 2005. The effect of growth rate on tissue-diet isotopic spacing in rapidly growing animals. An experimental study with Atlantic salmon (Salmo salar). Rapid Communications in Mass Spectrometry 19: 3239–3247.
Tyler, C. R. & J. P. Sumpter, 1996. Oocyte growth and development in teleosts. Reviews in Fish Biology and Fisheries 6: 287–318.
Vaudo, J. J., P. Matich & M. R. Heithaus, 2010. Mother–offspring isotope fractionation in two species of placentatrophic sharks. Journal of Fish Biology 77: 1724–1727.
Vincent, S. E., 2006. Sex-based divergence in head shape and diet in the Eastern lubber grasshopper (Romalea microptera). Zoology 109: 331–338.
Vincent, S. E., A. Herrel & D. J. Irschick, 2004. Sexual dimorphism in head shape and diet in the cottonmouth snake (Agkistrodon piscivorus). Journal of Zoology 264: 53–59.
Wearmouth, V. J. & D. W. Sims, 2008. Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms and conservation implications. Advances in Marine Biology 54: 107–170.
Acknowledgements
Financial and in-kind supports were provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) Strategic Grants and Discovery Grants Programs, Fisheries and Oceans Canada, and the Ontario Ministry of Natural Resources and Forestry. Field and laboratory support were provided by Lee Haslam, Tom Herra, Matthew Moles, Micale Prévost, Patricia Woloshyn, and Diana Wong. Constructive criticisms on earlier drafts of this manuscript were provided by Bob Montgomerie, Bethany Bethke, and one anonymous reviewer.
Author information
Authors and Affiliations
Contributions
TAJ, JMC, and WCL designed the study; TAJ led data collection and analysis; TAJ, JMC, and WCL wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involved in human or animal rights
This research was reviewed and approved by Animal Care Committees of Queen’s University and the Ontario Ministry of Natural Resources and Forestry.
Additional information
Handling editor: Michael Power
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Johnston, T.A., Casselman, J.M. & Leggett, W.C. Sex-based divergence in isotopic compositions of north temperate freshwater fishes. Hydrobiologia 848, 873–884 (2021). https://doi.org/10.1007/s10750-020-04496-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04496-4