Abstract
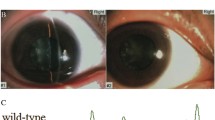
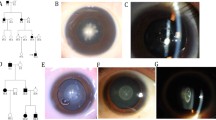
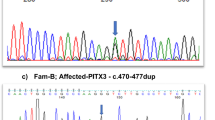
Peroxisomes, single-membrane intracellular organelles, play an important role in various metabolic pathways. The translocation of proteins from the cytosol to peroxisomes depends on peroxisome import receptor proteins and defects in peroxisome transport result in a wide spectrum of peroxisomal disorders. Here, we report a large consanguineous family with autosomal recessive congenital cataracts and developmental defects. Genome-wide linkage analysis localized the critical interval to chromosome 12p with a maximum two-point LOD score of 4.2 (θ = 0). Next-generation exome sequencing identified a novel homozygous missense variant (c.653 T > C; p.F218S) in peroxisomal biogenesis factor 5 (PEX5), a peroxisome import receptor protein. This missense mutation was confirmed by bidirectional Sanger sequencing. It segregated with the disease phenotype in the family and was absent in ethnically matched control chromosomes. The lens-specific knockout mice of Pex5 recapitulated the cataractous phenotype. In vitro import assays revealed a normal capacity of the mutant PEX5 to enter the peroxisomal Docking/Translocation Module (DTM) in the presence of peroxisome targeting signal 1 (PTS1) cargo protein, be monoubiquitinated and exported back into the cytosol. Importantly, the mutant PEX5 protein was unable to form a stable trimeric complex with peroxisomal biogenesis factor 7 (PEX7) and a peroxisome targeting signal 2 (PTS2) cargo protein and, therefore, failed to promote the import of PTS2 cargo proteins into peroxisomes. In conclusion, we report a novel missense mutation in PEX5 responsible for the defective import of PTS2 cargo proteins into peroxisomes resulting in congenital cataracts and developmental defects.







Similar content being viewed by others
References
Alencastre IS, Rodrigues TA, Grou CP, Fransen M, Sa-Miranda C, Azevedo JE (2009) Mapping the cargo protein membrane translocation step into the PEX5 cycling pathway. J Biol Chem 284:27243–27251
Baes M, Dewerchin M, Janssen A, Collen D, Carmeliet P (2002) Generation of Pex5-loxP mice allowing the conditional elimination of peroxisomes. Genesis 32:177–178
Baroy T, Koster J, Stromme P, Ebberink MS, Misceo D, Ferdinandusse S, Holmgren A, Hughes T, Merckoll E, Westvik J, Woldseth B, Walter J, Wood N, Tvedt B, Stadskleiv K, Wanders RJ, Waterham HR, Frengen E (2015) A novel type of rhizomelic chondrodysplasia punctata, RCDP5, is caused by loss of the PEX5 long isoform. Hum Mol Genet 24:5845–5854
Braverman NE, Moser AB (2012) Functions of plasmalogen lipids in health and disease. Biochim Biophys Acta 1822:1442–1452
Braverman N, Steel G, Obie C, Moser A, Moser H, Gould SJ, Valle D (1997) Human PEX7 encodes the peroxisomal PTS2 receptor and is responsible for rhizomelic chondrodysplasia punctata. Nat Genet 15:369–376
Braverman N, Dodt G, Gould SJ, Valle D (1998) An isoform of pex5p, the human PTS1 receptor, is required for the import of PTS2 proteins into peroxisomes. Hum Mol Genet 7:1195–1205
Braverman N, Chen L, Lin P, Obie C, Steel G, Douglas P, Chakraborty PK, Clarke JT, Boneh A, Moser A, Moser H, Valle D (2002) Mutation analysis of PEX7 in 60 probands with rhizomelic chondrodysplasia punctata and functional correlations of genotype with phenotype. Hum Mutat 20:284–297
Brocard C, Hartig A (2006) Peroxisome targeting signal 1: is it really a simple tripeptide? Biochim Biophys Acta 1763:1565–1573
Brosche T, Platt D (1998) The biological significance of plasmalogens in defense against oxidative damage. Exp Gerontol 33:363–369
Buchert R, Tawamie H, Smith C, Uebe S, Innes AM, Al HB, Ekici AB, Sticht H, Schwarze B, Lamont RE, Parboosingh JS, Bernier FP, Abou JR (2014) A peroxisomal disorder of severe intellectual disability, epilepsy, and cataracts due to fatty acyl-CoA reductase 1 deficiency. Am J Hum Genet 95:602–610
Carvalho AF, Grou CP, Pinto MP, Alencastre IS, Costa-Rodrigues J, Fransen M, Sa-Miranda C, Azevedo JE (2007) Functional characterization of two missense mutations in Pex5p - C11S and N526K. Biochim Biophys Acta 1773:1141–1148
Carvalho AF, Pinto MP, Grou CP, Alencastre IS, Fransen M, Sa-Miranda C, Azevedo JE (2007) Ubiquitination of mammalian Pex5p, the peroxisomal import receptor. J Biol Chem 282:31267–31272
Distel B, Erdmann R, Gould SJ, Blobel G, Crane DI, Cregg JM, Dodt G, Fujiki Y, Goodman JM, Just WW, Kiel JA, Kunau WH, Lazarow PB, Mannaerts GP, Moser HW, Osumi T, Rachubinski RA, Roscher A, Subramani S, Tabak HF, Tsukamoto T, Valle D, van der Klei I, Van Veldhoven PP, Veenhuis M (1996) A unified nomenclature for peroxisome biogenesis factors. J Cell Biol 135:1–3
Dodt G, Braverman N, Wong C, Moser A, Moser HW, Watkins P, Valle D, Gould SJ (1995) Mutations in the PTS1 receptor gene, PXR1, define complementation group 2 of the peroxisome biogenesis disorders. Nat Genet 9:115–125
Ebberink MS, Mooyer PA, Koster J, Dekker CJ, Eyskens FJ, Dionisi-Vici C, Clayton PT, Barth PG, Wanders RJ, Waterham HR (2009) Genotype-phenotype correlation in PEX5-deficient peroxisome biogenesis defective cell lines. Hum Mutat 30:93–98
Francisco T, Rodrigues TA, Freitas MO, Grou CP, Carvalho AF, Sa-Miranda C, Pinto MP, Azevedo JE (2013) A cargo-centered perspective on the PEX5 receptor-mediated peroxisomal protein import pathway. J Biol Chem 288:29151–29159
Francisco T, Rodrigues TA, Dias AF, Barros-Barbosa A, Bicho D, Azevedo JE (2017) Protein transport into peroxisomes: knowns and unknowns. BioEssays 39:1700047
Gatto GJ Jr, Geisbrecht BV, Gould SJ, Berg JM (2000) Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat Struct Biol 7:1091–1095
Gorgas K, Teigler A, Komljenovic D, Just WW (2006) The ether lipid-deficient mouse: tracking down plasmalogen functions. Biochim Biophys Acta 1763:1511–1526
Gouveia AM, Guimaraes CP, Oliveira ME, Sa-Miranda C, Azevedo JE (2003) Insertion of Pex5p into the peroxisomal membrane is cargo protein-dependent. J Biol Chem 278:4389–4392
Grou CP, Carvalho AF, Pinto MP, Huybrechts SJ, Sa-Miranda C, Fransen M, Azevedo JE (2009) Properties of the ubiquitin-pex5p thiol ester conjugate. J Biol Chem 284:10504–10513
Grou CP, Francisco T, Rodrigues TA, Freitas MO, Pinto MP, Carvalho AF, Domingues P, Wood SA, Rodriguez-Borges JE, Sa-Miranda C, Fransen M, Azevedo JE (2012) Identification of ubiquitin-specific protease 9X (USP9X) as a deubiquitinase acting on ubiquitin-peroxin 5 (PEX5) thioester conjugate. J Biol Chem 287:12815–12827
Hoang TV, Kumar PK, Sutharzan S, Tsonis PA, Liang C, Robinson ML (2014) Comparative transcriptome analysis of epithelial and fiber cells in newborn mouse lenses with RNA sequencing. Mol Vis 20:1491–1517
Kakrana A, Yang A, Anand D, Djordjevic D, Ramachandruni D, Singh A, Huang H, Ho JWK, Lachke SA (2018) iSyTE 2.0: a database for expression-based gene discovery in the eye. Nucleic Acids Res 46:D875–D885
Khan SY, Hackett SF, Lee MC, Pourmand N, Talbot CC Jr, Riazuddin SA (2015) Transcriptome profiling of developing murine lens through RNA sequencing. Invest Ophthalmol Vis Sci 56:4919–4926
Khan SY, Vasanth S, Kabir F, Gottsch JD, Khan AO, Chaerkady R, Lee MC, Leitch CC, Ma Z, Laux J, Villasmil R, Khan SN, Riazuddin S, Akram J, Cole RN, Talbot CC, Pourmand N, Zaghloul NA, Hejtmancik JF, Riazuddin SA (2016) FOXE3 contributes to peters anomaly through transcriptional regulation of an autophagy-associated protein termed DNAJB1. Nat Commun 7:10953
Khan SY, Ali M, Kabir F, Renuse S, Na CH, Talbot CC Jr, Hackett SF, Riazuddin SA (2018) Proteome profiling of developing murine lens through mass spectrometry. Invest Ophthalmol Vis Sci 59:100–107
Kunze M (2020) The type-2 peroxisomal targeting signal. Biochim Biophys Acta Mol Cell Res 1867:118609
Kurochkin IV, Mizuno Y, Konagaya A, Sakaki Y, Schonbach C, Okazaki Y (2007) Novel peroxisomal protease Tysnd1 processes PTS1- and PTS2-containing enzymes involved in β-oxidation of fatty acids. EMBO J 26:835–845
Lathrop GM, Lalouel JM (1984) Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 36:460–465
Luoma AM, Kuo F, Cakici O, Crowther MN, Denninger AR, Avila RL, Brites P, Kirschner DA (2015) Plasmalogen phospholipids protect internodal myelin from oxidative damage. Free Radic Biol Med 84:296–310
Marsili S, Salganik RI, Albright CD, Freel CD, Johnsen S, Peiffer RL, Costello MJ (2004) Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res 79:595–612
Miyata N, Fujiki Y (2005) Shuttling mechanism of peroxisome targeting signal type 1 receptor Pex5: ATP-independent import and ATP-dependent export. Mol Cell Biol 25:10822–10832
Motley AM, Hettema EH, Hogenhout EM, Brites P, ten Asbroek AL, Wijburg FA, Baas F, Heijmans HS, Tabak HF, Wanders RJ, Distel B (1997) Rhizomelic chondrodysplasia punctata is a peroxisomal protein targeting disease caused by a non-functional PTS2 receptor. Nat Genet 15:377–380
Neuhaus A, Kooshapur H, Wolf J, Meyer NH, Madl T, Saidowsky J, Hambruch E, Lazam A, Jung M, Sattler M, Schliebs W, Erdmann R (2014) A novel Pex14 protein-interacting site of human Pex5 is critical for matrix protein import into peroxisomes. J Biol Chem 289:437–448
Otera H, Okumoto K, Tateishi K, Ikoma Y, Matsuda E, Nishimura M, Tsukamoto T, Osumi T, Ohashi K, Higuchi O, Fujiki Y (1998) Peroxisome targeting signal type 1 (PTS1) receptor is involved in import of both PTS1 and PTS2: studies with PEX5-defective CHO cell mutants. Mol Cell Biol 18:388–399
Pan D, Nakatsu T, Kato H (2013) Crystal structure of peroxisomal targeting signal-2 bound to its receptor complex Pex7p-Pex21p. Nat Struct Mol Biol 20:987–993
Pedrosa AG, Francisco T, Bicho D, Dias AF, Barros-Barbosa A, Hagmann V, Dodt G, Rodrigues TA, Azevedo JE (2018) Peroxisomal monoubiquitinated PEX5 interacts with the AAA ATPases PEX1 and PEX6 and is unfolded during its dislocation into the cytosol. J Biol Chem 293:11553–11563
Platta HW, Grunau S, Rosenkranz K, Girzalsky W, Erdmann R (2005) Functional role of the AAA peroxins in dislocation of the cycling PTS1 receptor back to the cytosol. Nat Cell Biol 7:817–822
Pronicka E, Piekutowska-Abramczuk D, Ciara E, Trubicka J, Rokicki D, Karkucinska-Wieckowska A, Pajdowska M, Jurkiewicz E, Halat P, Kosinska J, Pollak A, Rydzanicz M, Stawinski P, Pronicki M, Krajewska-Walasek M, Ploski R (2016) New perspective in diagnostics of mitochondrial disorders: two years’ experience with whole-exome sequencing at a national paediatric centre. J Transl Med 14:174
Purdue PE, Zhang JW, Skoneczny M, Lazarow PB (1997) Rhizomelic chondrodysplasia punctata is caused by deficiency of human PEX7, a homologue of the yeast PTS2 receptor. Nat Genet 15:381–384
Rodrigues TA, Alencastre IS, Francisco T, Brites P, Fransen M, Grou CP, Azevedo JE (2014) A PEX7-centered perspective on the peroxisomal targeting signal type 2-mediated protein import pathway. Mol Cell Biol 34:2917–2928
Rodrigues TA, Grou CP, Azevedo JE (2015) Revisiting the intraperoxisomal pathway of mammalian PEX7. Sci Rep 5:11806
Rodrigues TA, Francisco T, Dias AF, Pedrosa AG, Grou CP, Azevedo JE (2016) A cell-free organelle-based in vitro system for studying the peroxisomal protein import machinery. Nat Protoc 11:2454–2469
Schaffer AA, Gupta SK, Shriram K, Cottingham RW (1994) Avoiding recomputation in genetic linkage analysis. Hum Hered 44:225–237
Shiels A, Hejtmancik JF (2015) Molecular genetics of cataract. Prog Mol Biol Transl Sci 134:203–218
Shiels A, Hejtmancik JF (2017) Mutations and mechanisms in congenital and age-related cataracts. Exp Eye Res 156:95–102
Shiels A, Hejtmancik JF (2019) Biology of inherited cataracts and opportunities for treatment. Annu Rev Vis Sci 5:123–149
Shimozawa N, Zhang Z, Suzuki Y, Imamura A, Tsukamoto T, Osumi T, Fujiki Y, Orii T, Barth PG, Wanders RJ, Kondo N (1999) Functional heterogeneity of C-terminal peroxisome targeting signal 1 in PEX5-defective patients. Biochem Biophys Res Commun 262:504–508
Srivastava SK, Ansari NH, Bhatnagar A (1990) Sugar induced cataractogenesis: a paradigm of oxidative tissue pathology? Lens Eye Toxic Res 7:161–171
Sullivan DT, Carroll WT, Kanik-Ennulat CL, Hitti YS, Lovett JA, Von KL (1985) Glyceraldehyde-3-phosphate dehydrogenase from Drosophila melanogaster. Identification of two isozymic forms encoded by separate genes. J Biol Chem 260:4345–4350
Thai TP, Rodemer C, Worsch J, Hunziker A, Gorgas K, Just WW (1999) Synthesis of plasmalogens in eye lens epithelial cells. FEBS Lett 456:263–268
van den Bosch H, Schutgens RB, Wanders RJ, Tager JM (1992) Biochemistry of peroxisomes. Annu Rev Biochem 61:157–197
Vang S, Corydon TJ, Borglum AD, Scott MD, Frydman J, Mogensen J, Gregersen N, Bross P (2005) Actin mutations in hypertrophic and dilated cardiomyopathy cause inefficient protein folding and perturbed filament formation. FEBS J 272:2037–2049
Wanders RJ (2014) Metabolic functions of peroxisomes in health and disease. Biochimie 98:36–44
Wanders RJ, Waterham HR (2006) Biochemistry of mammalian peroxisomes revisited. Annu Rev Biochem 75:295–332
Wanders RJ, Schumacher H, Heikoop J, Schutgens RB, Tager JM (1992) Human dihydroxyacetonephosphate acyltransferase deficiency: a new peroxisomal disorder. J Inherit Metab Dis 15:389–391
Wanders RJ, Dekker C, Hovarth VA, Schutgens RB, Tager JM, Van LP, Lecoutere D (1994) Human alkyldihydroxyacetonephosphate synthase deficiency: a new peroxisomal disorder. J Inherit Metab Dis 17:315–318
Zhao H, Yang Y, Rizo CM, Overbeek PA, Robinson ML (2004) Insertion of a Pax6 consensus binding site into the alphaA-crystallin promoter acts as a lens epithelial cell enhancer in transgenic mice. Invest Ophthalmol Vis Sci 45:1930–1939
Zhao Y, Wilmarth PA, Cheng C, Limi S, Fowler VM, Zheng D, David LL, Cvekl A (2019) Proteome-transcriptome analysis and proteome remodeling in mouse lens epithelium and fibers. Exp Eye Res 179:32–46
Acknowledgements
The authors are grateful to all the members for their participation in this study. The work was supported by National Eye Institute Grant R01EY022714 (SAR). Work in JEA Laboratory was financed by FEDER—Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020—Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT—Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior in the framework of the project PTDC/BEX-BCM/2311/2014 (POCI-01-0145-FEDER-016613) and the projects "Institute for Research and Innovation in Health Sciences" (POCI-01-0145-FEDER-007274) and NORTE-01-0145-FEDER-000008 -Porto Neurosciences and Neurologic Disease Research Initiative at I3S, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). TF and TAR are supported by Fundação para a Ciência e Tecnologia, Programa Operacional Potencial Humano do QREN, and Fundo Social Europeu.
Author information
Authors and Affiliations
Contributions
MA, SYK, SAR: conceived and designed the experiments; SR, MLR, MB, JEA, JFH, SAR: contributed reagents, materials, and analytical tools; MA, SYK, TAR, TF, FK, BI, and BR: performed experiments; AAK, AM, MAN, MZA, MHA, MS, SJK, JK, SR: enrolled human subjects and ascertained clinical evaluations; MA, SYK, TAR, TF, XJ, HQ, FK, KKA, SR, SR, MLR, MB, JEA, JFH, SAR: data analysis and critical review of the manuscript; MA, SYK, TAR, TF, SR, SR, MLR, MB, JEA, JFH, SAR: contributed to writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ali, M., Khan, S.Y., Rodrigues, T.A. et al. A missense allele of PEX5 is responsible for the defective import of PTS2 cargo proteins into peroxisomes. Hum Genet 140, 649–666 (2021). https://doi.org/10.1007/s00439-020-02238-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02238-z