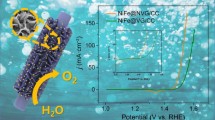
Abstract
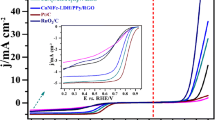
Self-supported non-noble metal based bifunctional electrocatalysts with high catalytic activity and long-term stability in a wide pH range are highly essential for the production of hydrogen and oxygen, remains a great challenge. Herein, a bifunctional electrocatalyst is synthesized via electroless plating of FeCoNiP nanoparticles on self-supported phosphorus-doped vertically aligned graphene arrays (FeCoNiP/P-VG). FeCoNiP/P-VG exhibits an exceptionally high catalytic activity for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) in a wide pH range, with an overpotential of 81 and 141 mV for HER, and 240 and 409 mV for OER, in 1.0 M KOH and 0.5 M H2SO4 respectively, at current density of 10 mA. It also performs quite low Tafel slope value of 40 mV·dec−1 for HER and 69 mV·dec−1 for OER in 1.0 M KOH. More importantly, it shows prominent stability in acidic and alkaline electrolytes. This study may open a new avenue for the design and fabrication of self-supported bifunctional electrocatalysts for water splitting.
Graphic Abstract










Similar content being viewed by others
References
Zhang, H.J., Li, X.P., Hähnel, A., Naumann, V., Lin, C., Azimi, S., Schweizer, S.L., Maijenburg, A.W., Wehrspohn, R.B.: Nanowires for highly efficient and stable overall water splitting. Adv. Funct. Mater. 28, 1706847 (2018)
Zhang, J.J., Lv, L.B., Zhao, T.J., Lin, Y.X., Yu, Q.Y., Su, J., Li, X.H., Chen, J.S.: Engineering the interfaces of super-absorbing graphene-based electrodes with gas and electrolyte to boost gas evolution/activation reactions. Chemsuschem 11, 2306–2309 (2018)
Anantharaj, S., Karthik, P.E., Subramanian, B., Kundu, S.: Pt nanoparticle anchored molecular self-assemblies of DNA: an extremely stable and efficient HER electrocatalyst with ultralow Pt content. ACS Catal. 6, 4660–4672 (2016)
Seh, Z.W., Kibsgaard, J., Dickens, C.F., Chorkendorff, I., Nørskov, J.K., Jaramillo, T.F.: Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017)
Huynh, M., Ozel, T., Liu, C., Lau, E.C., Nocera, D.G.: Design of template-stabilized active and earth-abundant oxygen evolution catalysts in acid. Chem. Sci. 8, 4779–4794 (2017)
Jiang, P., Liu, Q., Liang, Y.H., Tian, J.Q., Asiri, A.M., Sun, X.P.: A cost-effective 3D hydrogen evolution cathode with high catalytic activity: FeP nanowire array as the active phase. Angew. Chem. Int. Ed. 53, 12855–12859 (2014a)
Feng, L.G., Vrubel, H., Bensimon, M., Hu, X.L.: Phys, Easily-prepared dinickel phosphide (Ni2P) nanoparticles as an efficient and robust electrocatalyst for hydrogen evolution. Phys. Chem. Chem. Phys. 16, 5917–5921 (2014)
Tian, J.Q., Liu, Q., Asiri, A.M., Sun, X.P.: Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 136, 7587–7590 (2014a)
Li, R.Q., Wang, B.L., Gao, T., Zhang, R., Xu, C.Y., Jiang, X.F., Zeng, J.J., Bando, Y., Hu, P.F., Li, Y.L., Wang, X.B.: Monolithic electrode integrated of ultrathin NiFeP on 3D strutted graphene for bifunctionally efficient overall water splitting. Nona Energy 58, 870–876 (2019)
Liu, P., Rodriguez, J.A.: Catalysts for hydrogen evolution from the [NiFe] hydrogenase to the Ni2P (001) surface: the importance of ensemble effect. J. Am. Chem. Soc. 127, 14871–14878 (2005)
Stern, L.A., Feng, L., Song, F., Hu, X.L.: Ni2P as a janus catalyst for water splitting: the oxygen evolution activity of Ni2P nanoparticles. Energy Environ. Sci. 8, 2347–2351 (2015)
Tan, Y.W., Wang, H., Liu, P., Shen, Y.H., Cheng, C., Hirata, A., Fujita, T., Tang, Z., Chen, M.W.: Versatile nanoporous bimetallic phosphides towards electrochemical water splitting. Energy Environ. Sci. 9, 2257–2261 (2016)
Yu, J., Li, Q.Q.Y., Xu, C.Y., Zhen, L., David, V.P., Wu, J.S.: Ternary metal phosphide with triple-layered structure as a low-cost and efficient electrocatalyst for bifunctional water splitting. Adv. Funct. Mater. 26, 7644–7651 (2016)
Yan, G., Tan, H.Q., Wang, Y.H., Li, Y.G.: Amorphous quaternary alloy phosphide hierarchical nanoarrays with pagoda-like structure grown on Ni foam as pH-universal electrocatalyst for hydrogen evolution reaction. Appl. Surf. Sci. 489, 519–527 (2019)
Xu, J.Y., Li, J.J., Xiong, D.H., Zhang, B.S., Liu, Y.F., Wu, K., Amorim, I., Li, W., Liu, L.F.: Trends in activity for the oxygen evolution reaction on transition metal (M = Fe Co, Ni) phosphide pre-catalysts. Chem. Sci. 9, 3470–3476 (2018)
Cao, X.Y., Cui, L., Wang, X.X., Yang, W.R., Liu, J.Q.: Nickel-borate/reduced graphene oxide nanohybrid: a robust and efficient electrocatalyst for oxygen evolution reaction in alkaline and near neutral media. ChemCatChem. 10, 2826–2832 (2018)
Nsanzimana, J.M.V., Dangol, R., Reddu, V., Duo, S., Peng, Y.C., Dinh, K.N., Huang, Z.F., Yan, Q.Y., Wang, X.: Facile synthesis of amorphous ternary metal borides-reduced graphene oxide hybrid with superior oxygen evolution activity. ACS Appl. Mater. Interfaces 11, 846–855 (2019)
Huang, H., Wang, X.: Recent progress on carbon-based support materials for electrocatalysts of direct methanol fuel cells. J. Mater. Chem. A 2, 6266–6291 (2014)
Qiu, H.J., Guan, Y.X., Luo, P., Wang, Y.: Recent advance in fabricating monolithic 3D porous graphene and their applications in biosensing and biofuel cells. Biosens. Bioelectron. 89, 85–95 (2017)
Hong, J., Char, K., Kim, B.S.: Hollow capsules of reduced graphene oxide nanosheets assembled on a sacrificial colloidal particle. J. Phys. Chem. Lett. 24, 3442–3445 (2010)
Ahn, H.S., Kim, J.M., Park, C., Jang, J.W., Lee, J.S., Kim, H., Kaviany, M., Kim, M.H.: A novel role of three dimensional graphene foam to prevent heater failure during boiling. Sci Rep. 3, 1960 (2013)
Zhang, P.P., Li, J., Lv, L.X., Zhao, Y., Qiu, L.T.: Vertically aligned graphene sheets membrane for highly efficient solar thermal generation of clean water. ACS Nano 11, 5087–5093 (2017)
Gadipelli, S., Guo, Z.X.: Graphene-based materials: synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 69, 1–60 (2015)
Sadeghinezhad, E., Mehrali, M., Saidur, R., Mehrali, M., Latibari, S.T., Akhiani, A.R., Metselaar, H.S.C.: A comprehensive review on graphene nanofluids: recent research, development and applications. Energy Convers. Manage. 111, 466–487 (2016)
An, M., Du, C.Y., Du, L., Sun, Y.G., Wang, Y.J., Chen, C., Han, G.K., Yin, G.P., Gao, Y.Z.: Phosphorus-doped graphene support to enhance electrocatalysis of methanol oxidation reaction on platinum nanoparticles. Chem. Phys. Lett. 687, 1–8 (2017)
Peng, Z., Yu, Y., Jiang, D., Wu, Y., Xia, B.Y., Dong, Z.: N-doped carbon shell coated CoP nanocrystals encapsulated in porous N-doped carbon substrate as efficient electrocatalyst of water splitting. Carbon 144, 464–471 (2019)
He, P.L., Yu, X.Y., Lou, X.W.: Carbon-incorporated nickel-cobalt mixed metal phosphide nanoboxes with enhanced electrocatalytic activity for oxygen evolution. Angew. Chem. Int. Edit. 56, 3897–3900 (2017)
Li, Z.S., Zhang, L., Huang, X.M., Ye, L.T.: ShenLin, shape-controlled synthesis of Pt nanoparticles via integration of graphene and β-cyclodextrin and using as a noval electrocatalyst for methanol oxidation. Electrochim. Acta 121, 215–222 (2015)
Hummers, W.S., Offeman, R.E., Am, J.: Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958)
Sumi, V.S., Ameen Sha, M., Arunima, S.R., Shibli, S.M.A.: Development of a novel method of NiCoP alloy coating for electrocatalytic hydrogen evolution reaction in alkaline media. Electrochim. Acta 303, 67–77 (2019)
Mendoza-Garcia, A., Zhu, H.Y., Yu, Y.S., Li, Q., Zhou, L., Su, D., Kramer, M.J., Sun, S.H.: Controlled anisotropic growth of Co-Fe-P from Co-Fe-O nanoparticles. Angew. Chem. Int. Ed. 54, 9642–9645 (2015)
Zhang, G.W., Wang, B., Bi, J.L., Fang, D.Q., Yang, S.C.: Constructing ultrathin CoP nanomeshes by Er-doping for highly efficient bifunctional electrocatalysts for overall water splitting. J. Mater. Chem. A. 10, 5769–5778 (2019)
Cao, L.M., Hu, Y.W., Tang, S.F., Iijin, A., Wang, J.W., Zhang, Z.M., Lu, T.B.: Fe-CoP electrocatalyst derived from a bimetallic Prussian blue analogue for large-current-density oxygen evolution and overall water splitting. Adv. Sci. 5, 1800949 (2018)
Wu, A.P., Xie, Y., Ma, H., Tian, C.G., Gu, Y., Yan, H.J., Zhang, X.M., Yang, G.Y., Fu, H.G.: Integrating the active OER and HER components as the heterostructures for the efficient overall water splitting. Nano Energy 44, 353–363 (2018)
Wang, X.S., Xu, C.C., Jaroniec, M., Zheng, Y., Qiao, S.Z.: Anomalous hydrogen evolution behavior in high-pH environment induced by locally generated hydronium ions. Nat. Commun. 10, 4876 (2019)
Li, P.P., Zhao, R.B., Chen, H.Y., Wang, H.B., Wei, P.P., Huang, H., Liu, Q., Li, T.S., Shi, X.F., Zhang, Y.Y., Liu, M.L., Sun, X.P.: Recent advances in the development of water oxidation electrocatalysts at mild pH. Small 15(13), 1805103 (2019)
Li, D., Baydoun, H., Verani, C.N., Brock, S.L.: Efficient water oxidation using CoMnP nanoparticles. J. Am. Chem. Soc. 138, 4006–4009 (2016)
McCrory, C.C.L., Jung, S.H., Ferrer, I.M., Chatman, S.M., Peters, J.C., Jaramillo, T.F.: Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water Splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015)
Duan, J.J., Chen, S., Jaroniec, M., Qiao, S.Z.: Heteroatom-doped graphene-based materials for energy-relevant electrocatalytic processes. ACS Catal. 5, 5207–5234 (2015)
Cecilia, J.A., Infantes-Molina, A., Rodr´ıguez-Castell´on, E., Jim´enez-L´opez, A.: A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene. J. Catal. 263, 4–15 (2009)
Chung, D.Y., Jun, S.W., Yoon, G., Kim, H., Yoo, J.M., Lee, K.S., Kim, T., Shin, H., Sinha, A.K., Kwon, S.G., Kang, K., Hyeon, T., Sung, Y.E., Am, J.: Large-scale synthesis of carbon-shell-coated FeP nanoparticles for robust hydrogen evolution reaction electrocatalyst. J. Am. Chem. Soc. 139, 6669–6674 (2017)
Hao, S., Yang, L.B., Liu, D.N., Kong, R.M., Du, G., Asiri, A.M., Yang, Y.C., Sun, X.P.: Integrating natural biomass electro-oxidation and hydrogen evolution: using a porous Fe-doped CoP nanosheet array as a bifunctional catalyst. Chem. Commun. 53, 5710–5713 (2017)
Tia, X.Q., Li, Y.H., Xia, D., Sun, J.: Ultrafast and large scale preparation of superior catalyst for oxygen evolution reaction. J. Power Sources. 365, 320–326 (2017)
Du, C., Yang, L., Yang, F.L., Cheng, G.Z., Luo, W.: Nest-like NiCoP for highly efficient overall water splitting. ACS Catal. 7, 4131–4137 (2017)
Hu, F., Zhu, S.L., Chen, S.M., Li, Y., Ma, L., Wu, T.P., Zhang, Y., Wang, C.M., Liu, C.C., Yang, X.J., Song, L., Yang, X.W., Xiong, Y.J.: Amorphous metallic NiFeP: a conductive bulk material achieving high activity for oxygen evolution reaction in both alkaline and acidic media. Adv. Mater. 29, 1606570 (2017)
Zhang, T., Du, J., Xi, P.X., Xu, C.L.: Hybrids of cobalt/iron phosphides derived from bimetal-organic frameworks as highly efficient electrocatalysts for oxygen evolution reaction. ACS Appl. Mater. Interfaces 9, 362–370 (2017)
Liang, H.F., Gandi, A.N., Anjum, D.H., Wang, X.B., Schwingenschlögl, U., Alshareef, H.N.: Plasma-assisted synthesis of NiCoP for efficient overall water splitting. Nano Lett. 16, 7718–7725 (2016)
Hao, J.H., Yang, W.S., Zhang, Z., Tang, J.L.: Metal-organic frameworks derived CoxFe1-xP nanocubes for electrochemical hydrogen evolution. Nanoscale. 7, 11055–11062 (2015)
Han, A.L., Jin, S., Chen, H.L., Ji, H.X., Sun, Z.J., Du, P.G.: A robust hydrogen evolution catalyst based on crystalline nickel phosphide nanoflakes on three-dimensional graphene/nickel foam: high performance for electrocatalytic hydrogen production from pH 0–14. J. Mater. Chem. A. 3, 1941–1946 (2015)
Yan, L.T., Jiang, H.M., Xing, Y.L., Wang, Y., Liu, D., Gu, X., Dai, P.C., Li, L.J., Zhao, X.B.: J, nickel metal–organic framework implanted on graphene and incubated to be ultrasmall nickel phosphide nanocrystals acts as a highly efficient water splitting electrocatalyst. J. Mater. Chem. A. 6, 1682–1691 (2018)
Zheng, Y., Jiao, Y., Li, L.H., Xing, T., Chen, Y., Jaroniec, M., Qiao, S.Z.: Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 8, 5290–5296 (2014)
Tian, J.Q., Liu, Q., Asiri, A.M., Sun, X.P.: Self-supported nanoporous cobalt phosphide nanowire arrays: an efficient. J. Am. Chem. Soc. 136, 7587–7590 (2014b)
You, B., Jiang, N., Sheng, M.L., Gul, S., Yano, J., Sun, Y.J.: High-performance overall water splitting electrocatalysts derived from cobalt-based metal-organic frameworks. Chem. Mater. 27, 7636–7642 (2015)
He, S.Q., He, S.Y., Gao, F., Bo, X., Wang, Q.X., Chen, X.J., Duan, J.J., Zhao, C.: Ni2P@carbon core-shell nanorod array derived from ZIF-67-Ni: effect of phosphorization temperature on morphology, structure and hydrogen evolution reaction performance. Appl. Surf. Sci. 457, 933–941 (2018)
Pan, Y., Liu, Y., Zhao, J.C., Yang, K., Liang, J.L., Liu, D.D., Hu, W.H., Liu, D.P., Liu, Y.Q., Liu, C.G.: Monodispersed nickel phosphide nanocrystals with different phases: synthesis, characterization and electrocatalytic properties for hydrogen evolution. J. Mater. Chem. A. 3, 1656–1665 (2015)
He, S.Q., He, S.Y., Bo, X., Wang, Q.X., Zhan, F.P., Wang, Q.H., Zhao, C.: Porous Ni2P/C microrods derived from microwave-prepared MOF-74-Ni and its electrocatalysis for hydrogen evolution reaction. Mater. Lett. 231, 94–97 (2018)
Ma, L.B., Shen, X.P., Zhou, H., Zhu, G.X., Ji, G.Y., Chen, K.M.: CoP nanoparticles deposited on reduced graphene oxide sheets as an active electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 3, 5337–5343 (2015)
Xiao, X.F., He, C.T., Zhao, S.L., Li, J., Lin, W.S., Yuan, Z.K., Zhang, Q., Wang, S.Y., Dai, L.M., Yu, D.S.: A general approach to cobalt-based homobimetallic phosphide ultrathin nanosheets for highly efficient oxygen evolution in alkaline media. Energy Environ. Sci. 10, 893–889 (2017)
Zhang, Z., Hao, J.H., Yang, W.S., Tang, J.L.: Iron triad (Fe Co, Ni) trinary phosphide nanosheet arrays as high-performance bifunctional electrodes for full water splitting in basic and neutral conditions. RSC Adv. 6, 9647–9655 (2016)
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21902127).
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21902127).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Availability of data and material
All the datas and materials are able to be obtained from corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Z., Shaik, F., Liang, K. et al. Self-Supported Phosphorus-Doped Vertically Aligned Graphene Arrays Integrated with FeCoNiP Nanoparticles as Bifunctional Electrocatalysts for Water-Splitting Over a Wide pH Range. Electron. Mater. Lett. 17, 87–101 (2021). https://doi.org/10.1007/s13391-020-00260-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13391-020-00260-x