Abstract
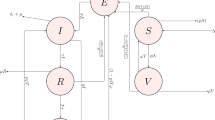
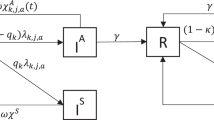
In this work, a co-infection model for human papillomavirus (HPV) and Chlamydia trachomatis with cost-effectiveness optimal control analysis is developed and analyzed. The disease-free equilibrium of the co-infection model is shown not to be globally asymptotically stable, when the associated reproduction number is less unity. It is proven that the model undergoes the phenomenon of backward bifurcation when the associated reproduction number is less than unity. It is also shown that HPV re-infection (\(\varepsilon_{\textsc{p}} \ne 0\)) induced the phenomenon of backward bifurcation. Numerical simulations of the optimal control model showed that: (i) focusing on HPV intervention strategy alone (HPV prevention and screening), in the absence of C. trachomatis control, leads to a positive population level impact on the total number of individuals singly infected with C. trachomatis, (ii) Concentrating on C. trachomatis intervention controls alone (C. trachomatis prevention and treatment), in the absence of HPV intervention strategies, a positive population level impact is observed on the total number of individuals singly infected with HPV. Moreover, the strategy that combines and implements HPV and C. trachomatis prevention controls is the most cost-effective of all the control strategies in combating the co-infections of HPV and C. trachomatis.












Similar content being viewed by others
References
Babaei A, Jafari H, Liya A (2020) Mathematical models of HIV/AIDS and drug addiction in prisons. Eur Phys J Plus 135:395
Blower SM, Dowlatabadi H (1994) Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev 2:229–243
Cantor SB, Ganiats TG (1999) Incremental cost-effectiveness analysis: the optimal strategy depends on the strategy set. Clin Epidemiol 52(6):517–522
Castillo-Chavez C, Song B (2004) Dynamical models of tuberculosis and their applications. Math Biosci Eng 2:361–404
Castillo-Chavez C, Feng Z, Huang W (1999) On the computation of \(R_{0}\) and its role on global stability. In: Mathematical approaches for emerging and reemerging infectious diseases: an introduction (Minneapolis, MN), pp 229–250, IMA Vol. Math. Appl., 125 Springer New York
Crawford B, Kribs-Zaleta CM (2009) The impact of vaccination and coinfection on HPV and cervical cancer. Discret Cont Dyn Syst Ser B 12(2):279–304
Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, Chlamydia - CDC Fact Sheet (Detailed), 2016, https://www.cdc.gov/std/chlamydia/stdfact-chlamydia-detailed.htm, Accessed 29 April 2020
Egonmwan AO, Okuonghae D (2019) Analysis of a mathematical model for tuberculosis with diagnosis. J Appl Math Comput 59:129–162. https://doi.org/10.1007/s12190-018-1172-1
Fleming WH, Rishel RW (1975) Deterministic and stochastic optimal control. Springer, New York
Gopalkrishna V, Aggarwal N, Malhotra VL (2000) Chlamydia trachomatis and human papillomavirus infection in Indian women with sexually transmitted diseases and cervical precancerous and cancerous lesions. Clin Microbiol Infect 6:88–93
Hethcote HW (2000) The mathematics of infectious diseases. SIAM Rev 42(4):599–653
Hussen S, Wachamo D, Yohannes Z, Tadesse E (2018) Prevalence of Chlamydia trachomatis infection among reproductive age women in sub Saharan Africa: a systematic review and meta-analysis. BMC Infect Dis. https://doi.org/10.1186/s12879-018-3477-y
Khan H, Gomez-Aguilar JF, Alkhazzan A, Khan A (2020) A fractional order HIV-TB coinfection model with nonsingular Mittag-Leffler Law. Math Method Appl Sci 1:21
Lakshmikantham S, Leela S, Martynyuk AA (1989) Stability analysis of nonlinear systems. Marcel Dekker Inc, New York
Lenhart S, Workman JT (2007) Optimal control applied to biological models. Chapman & Hall, Boca Raton
Lehmann M, Groh A, Rodel J, Nindl I, Straube E (1999) Detection of Chlamydia trachomatis DNA in cervical samples with regard to infection with human papillomavirus. J Infect 38:12–7
Malik T, Imran M, Jayaraman R (2016) Optimal control with multiple human papillomavirus vaccines. J Theor Biol 393:179–193
Mwamtobe PM, Simelane SM, Abelman S, Tchuenche JM (2018) Optimal control of intervention strategies in malariatuberculosis co-infection with relapse. Int J Biomath 11(2):1850017. https://doi.org/10.1142/S1793524518500171
Nonato DR, Alves RRF, Ribeiro AA, Saddi VA, Segati KD, Almeida KP, de Lima YAR, D’Alessandro WB, Rabelo-Santos SH (2016) Prevalence and factors associated with co-infection of human papillomavirus and Chlamydia trachomatis in adolescents and young women. Obstetr Gynecol Am J. https://doi.org/10.1016/j.ajog.2016.07.003
O’Farrell N, Morison L, Moodley P (2008) Genital ulcers and concomitant complaints in men attending a sexually transmitted infections clinic: implications for sexually transmitted infections management. Sex Trans Dis 35:545–9
Okosun KO, Makinde OD (2014) A co-infection model of malaria and cholera diseases with optimal control. Math Biosci 258:19–32
Okuonghae D (2019) Backward bifurcation of an epidemiological model with saturated incidence, isolation and treatment functions. Qual Theory Dyn Syst 18:413–440. https://doi.org/10.1007/s12346-018-0293-0
Okuonghae D, Omame A (2020) Analysis of a mathematical model for COVID-19 population dynamics in Lagos, Nigeria. Chaos Solitons Fractals 139:110032
Okuonghae D, Gumel AB, Ikhimwin BO, Iboi E (2019) Mathematical assessment of the role of early latent infections and targeted control strategies on syphilis transmission dynamics. Acta Biotheor 67:47–84. https://doi.org/10.1007/s10441-018-9336-9
Omame A, Umana RA, Okuonghae D, Inyama SC (2018) Mathematical analysis of a two-sex Human Papillomavirus (HPV) model. Int J Biomath 11(7):1850092
Omame A, Okuonghae D, Umana RA, Inyama SC (2020a) Analysis of a co-infection model for HPV-TB. Appl Math Model 77:881–901
Omame A, Okuonghae D, Inyama SC (2020b) A mathematical study of a model for HPV with two high risk strains. In: Smith F, Dutta H, Mordeson JN (eds) Mathematics applied to engineering, modelling, and social issues, studies in system, decision and control
Omame A, Sene N, Nometa I, Nwakanma CI, Nwafor EU, Iheonu NO, Okuonghae D (2020c) Analysis of COVID-19 and comorbidity co-infection Model with Optimal Control, medRxiv preprint https://doi.org/10.1101/2020.08.04.20168013
Paavonen J (2012) Chlamydia trachomatis infections of the female genital tract: state of the art. Ann Med 44(1):18–28. https://doi.org/10.3109/07853890.2010.546365
Pontryagin LS, Boltyanskii VG, Gamkrelidze RV, Mishchenko EF (1962) The mathematical theory of optimal processes. Wiley, New York
Saldana F, Korobeinikov A, Barradas I (2019) Optimal control against the human papillomavirus: protection versus eradication of the infection. Abstr Appl Anal. https://doi.org/10.1155/2019/4567825
Samanta GP (2015) Mathematical analysis of a chlamydia epidemic model with pulse vaccination strategy. Acta Biotheor 63:1–21
Samoff E, Koumans EH, Markowitz LE, Sternberg M, Sawyer MK, Swan D, Papp JR, Black CM, Unger ER (2005) Association of Chlamydia trachomatis with persistence of high-risk types of human papillomavirus in a cohort of female adolescents. Am J Epidemiol 162:668–675
Seraceni SS, Colli F, Del Savio C, Pesel R, Zanin G, D’Agarl V (2014) High prevalence of HPV multiple genotypes in women with persistent Chlamydia trachomatis infection. Infect Agents Canc 9(30):1–7
Sharomi O, Gumel AB (2009) Re-infection-induced backward bifurcation in the transmission dynamics of Chlamydia trachomatis. J Math Anal Appl 356:96–118
Sharomi O, Gumel AB (2011) Mathematical study of a risk-structured two-group model for Chlamydia transmission dynamics. Appl Math Model 35:3653–3673
Shew ML, Ermel AC, Weaver BA, Tong YTU, Kester W, Denski LM (2013) Association of Chlamydia trachomatis infection with redetection of human papillomavirus after apparent clearance. J Infect Dis 208:1416–21
Ssedyabane F, Amnia DA, Mayanja R, Omonigho A, Ssuuna C, Najjuma JN, Freddie B (2019) HPV-Chlamydial coinfection, prevalence, and association with cervical intraepithelial lesions: a pilot study at Mbarara Regional Referral Hospital. J Canc Epidem. https://doi.org/10.1155/2019/9092565
Silva J, Cerqueira F, Medeiros R (2014) Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Arch Gynecol Obstet 289:715–723. https://doi.org/10.1007/s00404-013-3122-3
Tanvi AR (2020) Dynamics of HIV-TB co-infection with detection as optimal intervention strategy. N Mech Int J. https://doi.org/10.1016/j.ijnonlinmec.2019.103388
Tanvi AR (2020) Stability analysis of a delayed HIV-TB co-infection model in resource limitation settings. Chaos Solitons Fractals 140:110138
Uganda Demographics Profile (2018) http://www.indexmundi.com/uganda/demographics_profile. Accessed 24 April 2020
Umana RA, Omame A, Inyama SC (2016) Deterministic and stochastic models of the dynamics of drug resistant tuberculosis. FUTO J Series 2(2):173–194
Uwakwe JI, Inyama SC, Omame A (2020) Mathematical model and optimal control of new-castle disease (ND). Appl Comput Math 9(3):70–84. https://doi.org/10.11648/j.acm.20200903.14
van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180:29–48
World Health Organization (2020) https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer. Accesssed 29 April 2020
Zhu H, Shen Z, Luo H, Zhang W, Zhu X (2016) Chlamydia trachomatis infection-associated risk of cervical cancer. A meta-analysis. Medicine 95(13):1–10
Acknowledgements
The authors are immensely grateful to the editors and anonymous reviewers for their invaluable and constructive criticisms, that have improved the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Dr. Andrew Omame, and all authors (including Mr. Nnanna U. Celestine and Prof. Simeon C. Inyama) commented on the previous version of the manuscript. All authors contributed in the work, and also read and approved the final revised manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Omame, A., Nnanna, C.U. & Inyama, S.C. Optimal Control and Cost-Effectiveness Analysis of an HPV–Chlamydia trachomatis Co-infection Model. Acta Biotheor 69, 185–223 (2021). https://doi.org/10.1007/s10441-020-09401-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-020-09401-z