Abstract
By 2050, the scale of installed solar panels must reach about 100 TWp in order to make a tangible impact on our energy mix and carbon emissions. Thin-film amorphous silicon panels are the only technology today capable of 100 TWp installation. Wafer silicon panels could reach 100 TWp if the silver in silicon panels is replaced with copper or aluminum. Cadmium telluride and copper indium gallium selenide would become technologies of insignificance in the big picture. For energy-efficient production of silicon panels, research is needed in energy-efficient purification of silicon, low kerf loss wafering of silicon, and an Earth-abundant top cell on silicon. Alternatively we can pursue a new cell technology which is more energy efficient than silicon and utilizes only Earth-abundant materials. For any cell technology, research is needed to improve the cost, efficiency, and sustainability including storage technologies for daily to multiyear storage and for regional and global trade of solar electricity, recycling technologies to minimize cost and maximize revenue from waste panels, and systems and applications for real-time and in situ consumption of solar electricity.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
There are three processes to utilize solar energy: solar to electrical, solar to chemical, and solar to thermal conversion. Photovoltaic devices convert sunlight into one of the two most popular energy carriers in our life, electricity. The other most popular energy carrier is fuel. Photoelectrochemical devices produce hydrogen as a fuel from sunlight and water (H2O). Solar energy can also be converted into thermal energy in solar water heaters for hot water and in concentrated solar power systems for electricity generation and chemical production. Out of these devices, only two are deployed commercially today: solar photovoltaic panels and solar water heaters, but solar water heaters do not produce electricity or fuel. Concentrated solar power systems are largely at the demonstration stage today, and photoelectrochemical devices are at the laboratory stage. This paper presents an analysis on some of the research needs for sustainable solar photovoltaics in the 21st century, as it is the only commercially-relevant solar technology today which generates electricity or fuel.
The photovoltaic effect was discovered in 1839 by Becquerel when he shed light onto a silver chloride (AgCl) electrode in an electrolyte. 1 The first silicon (Si) p-n junction solar cell was demonstrated in 1954 by Chapin, Fuller, and Pearson of Bell Laboratories. 2 The silicon wafer served as the light absorber to convert sunlight into electron-hole pairs and the internal electrical field in the p-n junction separated the electron-hole pairs. Although the efficiency of the first silicon cell was low at about 5% as compared to the current record efficiency of 26.7% for silicon cells, 3 the majority of today's commercial silicon cells still resemble the Bell Laboratories cell, i.e., they utilize a silicon wafer in either monocrystalline or multicrystalline form for light absorption and a p-n junction for charge separation.
The first application of solar cells was to power satellites which started in the 1950's. Terrestrial deployment of solar panels came about 20 years later after the 1973 oil embargo, which resulted in drastic oil price hikes. The motivation then for solar photovoltaics was more geopolitical than environmental. By the 1980's and 1990's the oil price had retreated and that motivation had eased since solar panels could not compete. 4 The heyday of solar photovoltaics began around 2005 when the oil price surged to $140/barrel. This time, more and more people recognized the environmental crisis we are facing by burning fossil fuels, and the push for solar photovoltaics has survived the fluctuations in oil price ever since. Meanwhile the phenomenal growth of the solar photovoltaics industry has driven down the cost and made solar panels financially viable.
Photovoltaics: 2000–2020
The solar photovoltaics industry has experienced phenomenal growth in the first 20 years of the 21st century. In 2000, the global cumulative installation of solar panels was 1.3 GWp (gigawatts peak) with an annual installation of 0.29 GWp. 5 By the end of 2020, the global cumulative installation of solar panels is expected to exceed 750 GWp with an annual installation close to 150 GWp. This is an over 500× increase or an average annual growth rate of about 37% for 20 years. Within the next 2 years, we will likely witness a milestone for the photovoltaics industry, i.e., a cumulative installation of 1 TWp (terawatt peak) of solar panels.
It is important to remember that, despite the 20-year phenomenal growth, solar electricity will remain a negligible source of energy for years to come. This is simply because of the enormous amount of energy we use. The global electricity capacity is currently over 7 TW. 6 Due to the intermittency of solar energy and the power losses in photovoltaic systems, the capacity factor of a solar panel is about 15%. Therefore, 750 GWp of solar panels is equivalent to an electricity capacity of about 110 GW, which is about 1.5% of the current global electricity capacity. Moreover, only about 20% of the energy we use is electricity; the rest is fuel. 6 That is, far less than 1% of the energy we use today comes from solar panels.
The rapid increase in solar electricity capacity will likely continue in the next few decades, maybe at a slower rate. During the same time, the global energy demand will no doubt increase. The current global average power demand is close to 20 TW. 7 That is, we use on average 20 TJ (terajoules) of energy every second. By 2050, the global power demand is projected to reach 30 TW. 8 If 50% of that power comes from solar panels, it translates to 15 TW of solar electricity. With a capacity factor of 15%, we are talking about 100 TWp of solar panels to be produced and installed in the next 30 years to meet half of the global energy demand by 2050. This is a 130× increase in solar electricity capacity from the 2020 level. If the remaining 50% of that demand is met by conventional energy sources such as coal, oil, and natural gas, we would reduce our conventional energy sources, and thus our carbon emissions, by about 25% from 20 TW today to 15 TW by 2050. Therefore, 100 TWp of solar panels is the minimum amount needed if solar electricity is going make a tangible impact on our carbon emissions. We will have to install either more than 100 TWp of solar panels or multiple low-carbon energy technologies to cut down our carbon emissions by, say, 30% from the 2020 level.
Why Solar Energy?
Solar energy is the only sustainable energy source with a sufficient capacity to meet all the global energy demand for the foreseeable future. Our planet receives about 1.2 × 105 TW of solar power, far exceeding our demand of 20 TW today and 30 TW by 2050. The solar energy our planet receives in a few hours is more than all the energy we use in an entire year. No other sustainable energy sources have such a capacity, so they can serve only as auxiliary energy sources in our future energy mix.
Other sustainable energy sources include wind, hydropower, biomass, ocean currents, tides and waves, and geothermal energy. It is interesting that most of the other sustainable energy sources originate from solar energy, except perhaps ocean tides and geothermal energy. The capacity of each of these non-solar sustainable energy sources is limited to about 1 TW, according to an analysis published in 2007. 9 That is, these non-solar sustainable energy sources are far short of the 20–30 TW scale required to become a primary energy source.
Instead of sustainable energy sources, we can also pursue low-carbon energy sources such as nuclear energy. There are two nuclear reactions which we can in principle tap into: fission and fusion. Nuclear fission is not the ultimate solution as its primary fuel, uranium, would be depleted in the not so distant future. 9 On the other hand, fission is a mature technology. It would take a long time to build up the solar electricity capacity for a tangible impact on our carbon emissions. The role fission could play in decarbonization is to buy us time to build up the solar capacity. Isotopes of hydrogen, deuterium and tritium, are the fuels for nuclear fusion, and there is enough of them on our planet to meet all the global energy demand for the foreseeable future. However, controlled fusion is required for power generation but it has yet to be demonstrated. Reference 10 provides an updated review on the status of nuclear fusion research by the World Nuclear Association. Commercial deployment of fusion reactors are tens of years away due to significant engineering challenges.
Photovoltaics: 2021–2050
It is difficult to predict the future, but one thing certain about solar photovoltaics is that the scale of installed panels must reach about 100 TWp by 2050 to make a tangible impact on our carbon emissions. The focus of photovoltaic research in the 20th century was cost and efficiency, which has enabled the commercial deployment of solar panels that we are witnessing. While cost and efficiency remain critically important for photovoltaic technologies, the 21st century will almost certainly encounter a number of new roadblocks to sustainable production and deployment of solar panels. 11 These roadblocks result from the sheer scale required for photovoltaics, which are unprecedented in other semiconductor technologies. We could accept low-efficiency high-cost solar panels if push comes to shove, but some of the new roadblocks we are going to face are more fundamental and could completely cripple the photovoltaics industry.
Raw material availability
Silver (Ag) is used in silicon solar cells as one of the two electrodes and as the soldering pads. Today each silicon cell requires about 70 mg (milligrams) of silver and generates 4.5–5 Wp of power depending on its dimension and efficiency. This is about 3 g of silver on a square meter of silicon solar cells. The known silver reserve on our planet, according to the U.S. Geological Survey, is 560,000 t (metric tons). 12 A simple calculation suggests that all the known silver reserve on our planet would allow a maximum of 36–40 TWp of silicon panels before the known silver reserve is depleted. This is far short of the 100 TWp needed by 2050.
There are four commercial solar panel technologies today. Besides silicon, the active layer in the solar cell can be cadmium telluride (CdTe), copper indium gallium selenide (CuInxGa1–xSe2 or CIGS), or amorphous silicon (a-Si). The market shares by different technologies are: silicon 95%, cadmium telluride 4%, copper indium gallium selenide 1%, and amorphous silicon 0.1%. 13 Cadmium telluride and copper indium gallium selenide suffer from the availability of tellurium and indium. Table I summarizes the maximum possible wattage for each of today's commercial panel technologies based on the reserve of the limiting material. 11 Amorphous silicon is the only technology capable of 100 TWp deployment, but it has been losing its market share for the last 20 years due to its low efficiency and high cost. An interesting question is: Would amorphous silicon come back if no other panel technology will reach 100 TWp? Today's silicon technology could be a noticeable source of energy by 2050, but it is incapable of reducing our carbon emissions. Between 2020 and 2050, the global power demand will increase by 10 TW, but 19 TWp of silicon panels would provide an equivalent electricity capacity of less than 3 TW. Cadmium telluride and copper indium gallium selenide would have to become technologies of insignificance in the big picture.
Table I. Maximum possible wattage for each commercial panel technology based on material availability. 11
| Technology | Efficiency | Limiting Material | Reserve (t) 10 | Maximum Wattage | Practical Wattage | % of 2050 Demand |
|---|---|---|---|---|---|---|
| Si | 19.0% | Ag | 560,000 | 38 TWp | 19 TWp | 9.5% |
| CdTe | 17.5% | Te | 31,000 | 0.8 TWp | 0.8 TWp | 0.4% |
| CIGS | 16.5% | In | 16,000 | 1.8 TWp | 0.9 TWp | 0.5% |
| a-Si | 9.0% | — | — | — | — | — |
Out of the three limiting materials in Table I, both silver and indium have important applications in other industries, so the photovoltaics industry does not have access to all the reserves. The practical wattage in Table I assumes that the photovoltaics industry could acquire half of the reserves. Excluding amorphous silicon, the remaining three technologies combined would provide a solar electricity capacity of about 20 TWp or an equivalent electricity capacity of 3 TW when fully deployed, which is about 10% of the 2050 global energy demand.
It is noted that the reserve data in Ref. 12 are estimates only and they are dependent on the price of the material. Price hikes for a material increase its reserve as hard-to-mine ores now become profitable. 14 Based on Table I, it would require a 50× more reserve of tellurium for cadmium telluride to provide 20% of the 2050 global energy demand, which is unlikely. For copper indium gallium selenide, a larger reserve of indium would not be sufficient as selenium is the next limiting material with a reserve of 99,000 t. 12 A 5× more reserve of silver would enable 100 TWp of silicon panels, which is more likely.
A higher material cost has a negative impact on the photovoltaics industry. For example, the silver price is $24.36/oz as of November 12, 2020. With 70 mg of silver per cell, the cost of silver material is about 6¢/cell today, without considering processing costs and profit margins. If the silver price goes back to its 2011 level at $46/oz, 15 the silver material cost would become 11.5¢/cell, i.e., an extra 5.5¢/cell. With the low profit margins in the photovoltaics industry (about 3¢/cell), the silver price alone could drive every silicon cell manufacturer into the red.
Energy intensity
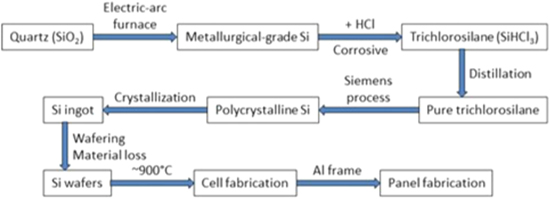
The production process for silicon solar panels is quite energy intensive, far more intensive than the other three panel technologies. Figure 1 shows the current industrial process to produce silicon panels from quartz. 11 It involves quartz reduction, silicon purification, ingot growth, wafering, cell fabrication, and panel fabrication. Overall it takes about 3 kWh of electricity to produce 1 Wp of silicon panels. 11 This sounds high, but it has seen an 80% reduction over the past 25 years as it was 16 kWh/Wp in the mid-1990's. 16 1 Wp of solar panels installed in Arizona generates close to 1.5 kWh/year, so the energy payback time for silicon panels is about 2 years in Arizona. In lower-insolation regions, the energy payback time can be twice as long.
Figure 1. The current industrial process to produce silicon solar panels from quartz. 11
Download figure:
Standard image High-resolution imageWith a 100 TWp total installation and a typical panel lifetime of 25 years, each year 4 TWp of panels would reach their end of life and we would need to produce 4 TWp/year to maintain a steady-state 100 TWp total installation. The production of 4 TWp/year of silicon panels would require 1.2 × 1013 kWh/year of electricity. This is equivalent of over 50% of the global electricity consumption in 2017. 6 This high energy intensity would not necessarily prevent us from eventually reaching 100 TWp of total installation, but it would certainly delay the deployment of silicon panels for a timely impact, depending on how much electricity we can allocate to the photovoltaics industry.
The most energy intensive step in silicon panel production is the Siemens process which reduces purified trichlorosilane (SiHCl3) to solar-grade silicon with at least a 99.9999% purity. 17 The second most energy intensive step is the ingot growth. 11 Multicrystalline silicon panels have a lower energy intensity than monocrystalline panels for two reasons. First, directional solidification for multicrystalline ingot growth is more energy-efficient than the Czochralski method for monocrystalline ingots. The former is a hot-wall reactor which can be thermally insulated from the surroundings to minimize thermal energy losses, while the latter is a cold-wall reactor which requires water cooling of the reactor walls. Second, rectangular multicrystalline ingots result in lower material loss during wafering than cylindrical monocrystalline ingots. The energy difference between multicrystalline and monocrystalline panels is about 1 kWh/Wp. 11
Traditionally our efforts to reduce the energy intensity of silicon panels have focused on:
- Improve the energy efficiency of the Siemens process;
- Reduce the consumption of the energy-intensive silicon material by thinner wafers and lower kerf loss;
- Improve the efficiency of silicon cells to increase the power output per gram of silicon;
- Produce multicrystalline silicon cells although they have lower efficiencies than monocrystalline cells.
Many of these methods are approaching their fundamental limitations. For example, the thickness of commercial silicon solar wafers has been stable at 180 μm for over 10 years now, 13 although research silicon cells have reached a wafer thickness of 50–100 μm. The difficulty with thinner wafers is that they are more likely to break during production, resulting in a lower yield. Another example is the energy intensity to produce silicon panels. It was 16 kWh/Wp in the mid-1990's. 16 15 years later (around 2010) it was down to about 4 kWh/Wp, 11 and after another 10 years it is now about 3 kWh/Wp. The pace of progress has slowed significantly.
Solar electricity storage
Storage is widely recognized as a roadblock to sustainable deployment of solar panels. With low solar penetration, the electric grid can serve as a buffer for intermittent solar electricity. Geographically-concentrated deployment of solar panels without storage in the last 20 years has created significant curtailment of solar electricity during peak solar production times in some regions. 18 Future large-scale photovoltaic systems will likely mandate storage. Today the default option for storage is battery. It is good for daily storage: electricity in during daytime and electricity out at night. If it is used for weekly storage (for example during a rainy week), the size of the battery needs to be increased by 7 times.
Long-term storage from monthly to seasonal to multiyear (for example the long dark winter in Sweden or the strategic petroleum reserve (SPR) in the U.S. 19 ) is difficult for battery, as the battery size would become prohibitively large. Chemical storage is a better option here. Hydrogen produced from water has been proposed as a storage medium for solar electricity. 20 It can be realized by coupling a photovoltaic system with a water electrolyzer. The downside of hydrogen storage is that it requires specialized tanks at an extremely low temperature and a high pressure to keep hydrogen in the liquid phase for a high energy density. There is also the risk of explosion.
Another possible storage medium is methanol (CH3OH) synthesized from hydrogen and carbon dioxide (CO2). 21 It involves multiple steps to capture carbon dioxide, generate hydrogen from renewable sources, and convert the two chemicals to methanol. It requires a more complicated process with a higher cost than hydrogen production alone. On the other hand, methanol could utilize the infrastructure for gasoline for its storage, distribution, and application as it is a liquid at room temperature with a boiling point of 65 °C.
An important aspect of fossil fuels is that they enable regional and global trade of energy. 22 Today many countries buy oil from the Middle East through oil tankers and Europe imports natural gas from Russia through a pipeline. Chemical storage enables regional and global trade of solar electricity. Both hydrogen and methanol are good for regional trade as they can be transported through pipelines, say, from Arizona to Chicago. Global trade of hydrogen, for example from Arizona to Sweden, requires specialized tankers to keep hydrogen in the liquid phase, but trading methanol globally could utilize oil tankers.
Solar panel recycling
Solar panels are not a closed-loop technology yet, but a circular economy requires recycling of waste solar panels so they are truly green and sustainable. Today most waste panels end up in landfills and some are sent to poor countries where electronic wastes are not regulated. According to the International Renewable Energy Agency, there would be 78 Mt (million metric tons) of waste panels cumulative by 2050 or 6 Mt/year in 2050. 23 This is likely an underestimate as the 2019 panel production was about 130 GWp 13 or about 8 Mt. With a typical panel lifetime of 25 years, these 2019 panels would become waste in 2044. By 2050, waste panels are likely to exceed 10 Mt/year.
There are more reasons to recycle solar panels besides just being green. 24 At the current mining rate of 27,000 t/year globally for silver, 12 the known silver reserve would be depleted in 21 years. We must recover silver from waste panels to sustain the silicon photovoltaics industry, until a silver-free silicon panel technology emerges. Silicon panels also contain lead, which is toxic, and fluoropolymer, which does not decompose when landfilled. Other materials contained in silicon panels include glass, aluminum, copper, tin, and of course silicon.
While more and more public attention is drawn to the important issue of solar panel recycling, it is rarely practiced today in most countries because the cost of recycling far exceeds the revenue from recycling. Today's recycling technology recovers only the glass, aluminum, and copper from waste silicon panels for a revenue of about $3/panel, but the cost is over $30/panel in the U.S. 24 We need to figure out a way to pay for solar panel recycling.
In the European Union, PV CYCLE 25 is one of the organizations set up to manage solar panel recycling. An upfront fee is charged to panel manufacturers to cover the recycling cost. In the U.S., the Solar Energy Industries Association started a voluntary solar panel recycling program in 2016, 26 paid for by panel owners.
Strategic R&D Directions
It is ironic that while we pursue solar photovoltaics for sustainability, almost all of the photovoltaic technologies we have today are unsustainable one way or another. Sustainability requires not only a sustainable energy source but also a sustainable technology to utilize that energy source. The latter has been largely overlooked in the 20th century. We all agree that the endgame for photovoltaic technologies is to cut down our carbon emissions. To achieve the goal, several strategic directions for photovoltaic research must be pursued now before they slow down or even stop the production and deployment of solar panels.
Earth-abundant materials
Any raw material used in photovoltaic technologies must have a large reserve, enough to support tens of terawatts peak of solar panels. This requirement results in a reduced Periodic Table of Earth-abundant elements as shown in Table II. 27 Out of the 110 elements in the Periodic Table, only 27 of them are Earth-abundant. The reserve data can be found in Ref. 12. The cutoff for Table II, 20 Mt, is somewhat arbitrary as the reserve needed for a material depends on the consumption per watt peak of that material in a panel technology. However, the message of Table II is clear: Only a small fraction of the elements in the Periodic Table are Earth-abundant and photovoltaic materials must come from a table similar to Table II.
Table II. A reduced Periodic Table of Earth-abundant elements in the upper continental crust. The cutoff is 20 Mt known reserve.
| IA | IIA | IVB | VB | VIB | VIIB | VIIIB | VIIIB | IB | IIB | IIIA | IVA | VA | VIA | VIIA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | ||||||||||||||
| B | C | N | O | F | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | ||||||||
| K | Ca | Ti | V | Cr | Mn | Fe | Ni | Cu | Zn | Br | ||||
| Zr | ||||||||||||||
| Ba | Pb |
If the scale of silicon solar panels is going to reach 100 TWp, we have to either find 5× more silver reserve on our planet, which is not impossible, or substitute silver with an Earth-abundant and low-resistivity material. There are only two metals which are suitable candidates for silver based on material abundance and resistivity, copper and aluminum. 11 Low cost is an added benefit by substituting silver with copper or aluminum, as silver is the second most-expensive component in silicon cells after the silicon wafer.
Research on substituting silver with copper as the front electrode on silicon solar cells is well underway, and high-efficiency cells with copper have been demonstrated. 28 The copper electrode requires a nickel barrier layer with silicon as copper is a deep-level impurity in silicon and kills the cell efficiency. 29 It also requires a tin capping layer to protect copper from oxidation. The three-layer electrode, nickel/copper/tin, is all electroplated. 30 Meanwhile there are efforts to fabricate the copper front electrode with a paste, 31 similar to the silver paste in today's cell fabrication. Aluminum as the front electrode on silicon cells does not require a barrier layer. 32 It is also possible to electroplate aluminum directly on silicon. 33 However, a high efficiency has yet to be demonstrated for silicon cells with an aluminum front electrode.
Alternatively, we can pursue a completely new solar cell technology which utilizes only Earth-abundant elements, but there is no guarantee that such a technology exists. Table II provides some hints about how to find an Earth-abundant semiconductor, if it exists, for a new cell technology. Except silicon, none of the elements in Table II is a semiconductor. Among the compounds, an alloy of several metals does not produce a semiconductor. Metal halides are usually not semiconductors. Semiconducting metal carbides, nitrides, and oxides typically have bandgaps too large for a high-efficiency cell. Therefore, the semiconductors to explore for a new cell technology should be metal silicides, phosphides, sulfides, or compounds between carbides, nitrides, oxides, silicides, phosphides, and sulfides.
Reference 34 compiles a list of metal chalcogenides as the active layer in a new solar cell technology including binary and quaternary sulfides and selenides of iron, copper, copper iron tin, copper manganese tin, copper barium tin, and copper nickel tin. Reference 35 reviews kesterites, i.e., quaternary copper zinc tin sulfide and selenide, for solar cells. Although not listed in Table II, there is probably enough tin on our planet, with a known reserve of 4.7 Mt, 12 for thin-film solar cells. The thickness of the active layer in thin-film cells is on the order of 1 μm, so the material consumption is several orders of magnitude smaller than silicon. The availability of selenium is limited with a known reserve of 99,000 t. 12
More complicated compound semiconductors are also possible for a new cell technology such as perovskites. 36 The progress in perovskite cell efficiency has been impressive from about 3% in 2006 to over 25% in 2020. 37 The most successful perovskite today for solar cells is methylammonium lead triiodide ((CH3NH3)PbI3) which contains lead and iodine. The more general formula of perovskites is alkylammonium lead halide (RHN3PbX3). 38 Would a perovskite panel technology be sustainable? The answer relies on multiple factors:
- The known reserve of iodine is 6.3 Mt. 12 What is the maximum possible wattage for perovskite panels based on the reserve of iodine?
- Perovskites are often sensitive to moisture, light, and heat. Can perovskite panels last 25 years?
- Lead is toxic. Even though the amount is small, how can we prevent lead from leaking out of a panel when the panel is damaged in the field?
- How can we recycle perovskite panels and recover all the components in the active layer: methylammonium, lead, and iodine?
It is recognized that perovskite cells are thin-film cells with an active layer thickness of less than 1 μm so material consumption is small. Perovskites also have a higher tolerance for defects which lends them to low-cost solution-based fabrication processes making them scalable. Finally, the amount of energy required to produce perovskite panels would be much lower than silicon panels.
Energy-efficient production
Traditional methods to reduce the energy intensity of silicon solar panels are approaching their fundamental limitations, but there are new methods to pursue. One potential method to improve the cell efficiency is a silicon tandem cell. There is not much room left for efficiency improvement in single-junction silicon cells. The Shockley–Queisser limit for single-junction cells is 31%, and the record efficiency for single-junction silicon cells is 26.7%. 39 A multi-junction tandem cell can exceed the 31% Shockley–Queisser limit. An Earth-abundant semiconductor with a suitable bandgap must be discovered to serve as the top cell on silicon. The ideal bandgap for the top cell on silicon is 1.74 eV (electron volts) for current matching between the top cell and the bottom silicon cell, but a bandgap down to about 1.6 eV can still be used. Perovskites provide an opportunity as the top cell on silicon, 40 with a record efficiency of over 29% now. 37
Thinner wafers result in a lower yield during cell and panel production due to wafer breakage. Kerf loss is determined largely by the diameter of the saw wire for wafering, and a thinner wire more readily breaks during cutting. One method to eliminate kerf loss is to crystallize silicon into a sheet form directly from a silicon melt, 41 bypassing ingot growth and wafering. The so-called ribbon silicon has not gained a foothold in the photovoltaics industry as it does not produce as high-efficiency cells due to its higher density of defects than silicon wafers from an ingot. Another method is to produce silicon wafers from an ingot without sawing. Implantation of hydrogen ions into a silicon ingot coupled with post-implantation annealing cleaves a sheet of silicon from an ingot. 42 However, large-area ion implantation is too expensive and too slow for the photovoltaics industry.
A much more energy-efficient process for silicon purification is desirable. In this aspect, electrorefining is attractive. 43 The Siemens process relies on fractional distillation for purification; a process based on the different boiling points of impurity chlorides and trichlorosilane. There are two steps in fractional distillation. In the first step, the vaporization temperature is controlled to keep all the impurity chlorides with boiling points higher than trichlorosilane in the liquid phase. In the second step, the vapor from the first step is condensed and the condensation temperature is controlled to keep all the impurity chlorides with boiling points lower than trichlorosilane in the gas phase. Electrorefining relies on a different material property for purification, i.e., the different redox potentials between impurities and silicon. It is in principle capable of producing ultrapure solar-grade silicon. However, the highest purity so far for electrorefined silicon is not enough for a high-efficiency cell. 44
Storage technologies
The focus of storage research has been on batteries, 45 and we are unprepared for chemical storage of solar electricity. Hydrogen storage requires a water electrolyzer and a photovoltaic array with a direct-current/direct-current power converter in between, but we do not have all the necessary components for such a solar electrolyzer. For example, the largest polymer electrolyte membrane electrolyzer today for hydrogen production from water is 2 MW, 46 but the largest direct-current/direct-current power converter is only 500 kW. 47 The power converter outputs 500 V and 1,000 A for battery charging, which mismatches the required input of the electrolyzer, 200 V and 10,000 A.
The storage medium, either in a battery or as a chemical, must be Earth-abundant. That is, the storage medium must come from Table II or even a subset of Table II as a 20 Mt reserve is likely insufficient as a storage medium. Hydrogen is in Table II. All the elements in methanol (CH3OH) are also in Table II. On the other hand, lithium is not in Table II.
Most of the elements in Table II are metals which could also serve as storage media. Similar to hydrogen, several of the metals are produced from their oxides by electrolysis. 48 The produced metal rods can be inserted into metal/air batteries as the anodes to generate electricity on demand, during which the metals oxidize. 49 The spent anodes must be shipped back for regeneration of the metal. The advantage of metals as storage media over hydrogen is that they are easy to store and transport. Among all the metals in Table II, the zinc/zinc oxide (Zn/ZnO) loop has been proposed as the best overall pick based on an analysis of four factors: material abundance, theoretical performance, process practicality, and technical readiness. 11
Table III compares the three possible storage media for solar electricity. Hydrogen is difficult to store and transport. Methanol requires a multi-step process to synthesize. Spent zinc anodes must be shipped back for recycling, while nature cycles back the products of hydrogen and methanol usage: water and carbon dioxide. None of the options in Table III are ideal but each offers its own advantages. A technoeconomic analysis can help quantify the pros and cons of each option.
Table III. Comparison of three possible storage media for solar electricity.
| Storage loop | Production process | Storage & transport | Return required |
|---|---|---|---|
| H2/H2O | One step | Difficult | No |
| CH3OH/CO2 + H2O | Multi-step | Moderate | No |
| Zn/ZnO | One step | Easy | Yes |
Recycling technologies
The high cost for solar panel recycling is partially attributed to the small quantity of waste panels today. If the panels last 25 years as promised by the manufacturers, we would now recycle panels installed in 1995. The annual installation in 1995 was about 77 MWp which is almost 2,000× less than the 2020 installation. The economy of scale has yet to kick in. On top of that, many waste panels are scattered geographically in small numbers. Retrieving a few panels hundreds of miles away is costly, and the collection cost currently dominates the recycling cost. Collection of waste panels requires reverse logistics.
The focus of recycling research should be on technologies which maximize the revenue from recycling and minimize the cost for recycling. There are three scenarios for decommissioned panels: panel reuse, component reuse, or material reuse. 24 If a decommissioned panel is still functional, it could be reused as a lower-quality product. It is noted that the typical degradation rate in panel efficiency is about 0.5%/year, so after a 30-year span the panel still produces about 85% of the original power. If the panel is not reusable due to damage or low efficiency, the components in the panel could be extracted for reuse including the silicon cells and the glass pane. If the components are not reusable, the materials in the components could be extracted for reuse including silver, lead, tin, copper, silicon, and glass. Out of the three scenarios, panel reuse generates the highest revenue with the fewest processing steps, while material reuse leads to the lowest revenue with the most processing steps. 24 However, all the reused panels and components will eventually fail and require material extraction. More importantly, any of the three scenarios would generate a much higher revenue than today's technology.
For component reuse and material reuse, a critical technology needed is to cleanly and gently separate the silicon cells from the glass pane. 24 This enables the silicon cells and glass pane to be reused. It also allows material extraction from the cells. Two low-concentration metals must be recovered from silicon cells: silver as a scarce material and lead as a toxic material. Their recovery requires chemical methods, while today's technology utilizes physical methods to recover bulky materials including glass, aluminum, and copper. Silver is the most valuable material in silicon panels, worth about $4/panel at a silver price of $24.36/oz as of November 12, 2020.
All the metals recovered from silicon panels can be sold directly on the commodity market including silver, aluminum, copper, tin, and lead. Selling the recovered glass cullet and silicon is a little tricky. 24 The recovered glass must be high-transparency solar glass cullet, or it could flood the market for low-quality glass cullet as there would millions of metric tons of this stuff each year from waste panels. Recovering high-purity solar-grade silicon is difficult, but the silicon wafer with a moderate-purity of at least 99.99% can be easily recovered. There is currently no market for silicon of this purity, so it has to be sold as low-purity metallurgical-grade silicon (99%). This is so unless a new application for this silicon can be found such as an electrode material for lithium ion batteries.
Applications of solar electricity
Systems and applications that accommodate the nature of solar electricity should be pursued. Industrialization got us into the habit that whenever and wherever we needed energy, it had to be there. On the contrary, our ancestors lived a different way. When the Sun was out, they went out and worked. When the Sun was gone, they remained in their caves and did little. Maybe we should adjust our lifestyles a bit based on the solar diurnal cycle as our ancestors did: when the Sun is out, we use more energy. When the Sun is gone, we use less energy. Solar electricity is distributed and intermittent. Possible approaches to accommodate the nature of solar electricity include:
- In situ consumption to take advantage of the geographic distribution of solar electricity: We can locally produce food, water, raw materials, or charge electric vehicles using solar electricity. This way a significant portion of the solar electricity does not become a burden to the electric grid or require chemical storage and transport.
- Real-time consumption to accommodate the intermittency of solar electricity: We can develop photovoltaic systems which take only supplemental power from the electric grid for backup. These solar-powered grid-backed systems are more likely to be adopted today as they improve the capacity factor and reduce the need for storage. In the long run, the grid must become clean for these systems to be 100% clean.
These concepts are broader than the concept of self-consumption of solar electricity, which involves storage so solar electricity can be used at a later time in the same building for self-sufficiency. 50 For in situ and real-time consumption of solar electricity, the system may or may not contain storage but focuses on local and real-time consumption to minimize storage.
In situ and real-time consumption could expand the applications of solar electricity into the manufacturing and transportation sectors. The conventional wisdom for solar-powered manufacturing and transportation is to make the electric grid cleaner: Direct-current solar electricity is first converted to alternating-current power and then feeds into the grid, where it is mixed with "dirty" power derived from fossil fuels. Manufacturers and vehicle owners take in this clean/dirty mixed power from the grid for their production and vehicle charging. As the grid gets cleaner, so does the manufacturing and transportation. In this scenario, manufacturers and vehicle owners are passive participants in fossil to solar energy transition. In situ and real-time consumption of solar electricity as described above enables manufacturers and vehicle owners to adopt solar electricity independent of the electric grid and its constraints.
Conclusions
The scale of installed solar panels needs to reach about 100 TWp by 2050 to reduce our carbon emissions by about 25% from the 2020 level. Out of the four commercial panel technologies we have today:
- Amorphous silicon is the only technology capable of 100 TWp deployment.
- Cadmium telluride and copper indium gallium selenide would become technologies of insignificance in the big picture.
- Silicon could reach 100 TWp if we succeed in finding 5× more silver on our planet or substituting silver with copper or aluminum in silicon cells.
- Or we can pursue a completely new cell technology based on an Earth-abundant metal silicide, phosphide, sulfide, or more complicated compound.
For any cell technology, there are several common topics to improve the cost, efficiency, and sustainability:
- Improvement in solar cell efficiency.
- Reduction in photovoltaic system cost.
- Storage technologies for daily to multiyear storage and for regional and global trade of solar electricity.
- Recycling technologies to minimize cost and maximize revenue from waste panels.
- Systems and applications for real-time and in situ consumption of solar electricity.
For the silicon panel technology, the following topics are also important for energy-efficient production:
- Energy-efficient purification of silicon.
- Low kerf loss wafering of silicon.
- An Earth-abundant top cell on silicon.
Without major technological breakthroughs in these topics, all the current commercial panel technologies combined would hardly make a dent in our energy mix or carbon emissions.