Abstract
Background
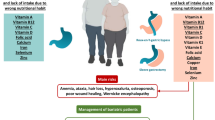
Chronic kidney disease (CKD) in children is a pro-inflammatory condition leading to a high morbidity and mortality. Accumulation of organic metabolic waste products, coined as uraemic toxins, parallels kidney function decline. Several of these uraemic toxins are protein-bound (PBUT) and gut-derived. Gut dysbiosis is a hallmark of CKD, resulting in a state of increased proteolytic fermentation that might be counteracted by dietary fibre. Data on fibre intake in children with CKD are lacking. We aimed to assess dietary fibre intake in a paediatric CKD cohort and define its relationship with PBUT concentrations.
Methods
In this multi-centre, cross-sectional observational study, 61 non-dialysis CKD patients (9 ± 5 years) were included. Dietary fibre intake was assessed through the use of 24-h recalls or 3-day food records and coupled to total and free levels of 4 PBUTs (indoxyl sulfate (IxS), p-cresyl sulfate (pCS), p-cresyl glucuronide (pCG) and indole acetic acid (IAA).
Results
In general, fibre intake was low, especially in advanced CKD: 10 ± 6 g/day/BSA in CKD 4–5 versus 14 ± 7 in CKD 1–3 (p = 0.017). Lower concentrations of both total (p = 0.036) and free (p = 0.036) pCG were observed in the group with highest fibre intake, independent of kidney function.
Conclusions
Fibre intake in paediatric CKD is low and is even worse in advanced CKD stages. Current dietary fibre recommendations for healthy children are not being achieved. Dietary management of CKD is complex in which too restrictive diets carry the risk of nutritional deficiencies. The relation of fibre intake with PBUTs remains unclear and needs further investigation.
Graphical abstract

Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Mitsnefes MM (2012) Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23:578–585. https://doi.org/10.1681/asn.2011111115
Tong A, Wong G, McTaggart S, Henning P, Mackie F, Carroll RP, Howard K, Craig JC (2013) Quality of life of young adults and adolescents with chronic kidney disease. J Pediatr 163:1179–1185.e1175. https://doi.org/10.1016/j.jpeds.2013.04.066
Ingelfinger JR, Kalantar-Zadeh K, Schaefer F (2016) Averting the legacy of kidney disease - focus on childhood. Kidney Dis (Basel) 2:46–52. https://doi.org/10.1159/000443819
Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, Kanbay M, Mallamaci F, Parati G, Rossignol P, Wiecek A, London G (2017) The systemic nature of CKD. Nat Rev Nephrol 13:344–358. https://doi.org/10.1038/nrneph.2017.52
Gryp T, Vanholder R, Vaneechoutte M, Glorieux G (2017) p-Cresyl sulfate. Toxins (Basel) 9:52. https://doi.org/10.3390/toxins9020052
Evenepoel P, Poesen R, Meijers B (2017) The gut-kidney axis. Pediatr Nephrol 32:2005–2014. https://doi.org/10.1007/s00467-016-3527-x
Vanholder R, Pletinck A, Schepers E, Glorieux G (2018) Biochemical and clinical impact of organic uremic retention solutes: a comprehensive update. Toxins (Basel) 10:33. https://doi.org/10.3390/toxins10010033
Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW (2011) Colonic contribution to uremic solutes. J Am Soc Nephrol 22:1769–1776. https://doi.org/10.1681/asn.2010121220
Mair RD, Sirich TL, Plummer NS, Meyer TW (2018) Characteristics of colon-derived uremic solutes. Clin J Am Soc Nephrol 13:1398–1404. https://doi.org/10.2215/cjn.03150318
Vaziri ND, Zhao YY, Pahl MV (2016) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant 31:737–746. https://doi.org/10.1093/ndt/gfv095
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS (2016) Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis 67:483–498. https://doi.org/10.1053/j.ajkd.2015.09.027
Vanholder R, Glorieux G (2015) The intestine and the kidneys: a bad marriage can be hazardous. Clin Kidney J 8:168–179. https://doi.org/10.1093/ckj/sfv004
Meijers B, Farre R, Dejongh S, Vicario M, Evenepoel P (2018) Intestinal barrier function in chronic kidney disease. Toxins (Basel) 10:298. https://doi.org/10.3390/toxins10070298
Gryp T, Huys GRB, Joossens M, Van Biesen W, Glorieux G, Vaneechoutte M (2020) Isolation and quantification of uremic toxin precursor-generating gut bacteria in chronic kidney disease patients. Int J Mol Sci 21:1986. https://doi.org/10.3390/ijms21061986
Vaziri ND (2016) Effect of synbiotic therapy on gut-derived uremic toxins and the intestinal microbiome in patients with CKD. Clin J Am Soc Nephrol 11:199–201. https://doi.org/10.2215/cjn.13631215
Poesen R, Meijers B, Evenepoel P (2013) The colon: an overlooked site for therapeutics in dialysis patients. Semin Dial 26:323–332. https://doi.org/10.1111/sdi.12082
Evenepoel P, Meijers BK, Bammens BR, Verbeke K (2009) Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114:S12–S19. https://doi.org/10.1038/ki.2009.402
Kieffer DA, Martin RJ, Adams SH (2016) Impact of dietary fibers on nutrient management and detoxification organs: gut, liver, and kidneys. Adv Nutr 7:1111–1121. https://doi.org/10.3945/an.116.013219
Cases A, Cigarran-Guldris S, Mas S, Gonzalez-Parra E (2019) Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients 11:1263. https://doi.org/10.3390/nu11061263
National Kidney Foundation (2009) KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 53(3 Suppl 2):S11–S104. https://doi.org/10.1053/j.ajkd.2008.11.017
Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. https://doi.org/10.1681/asn.2008030287
Snauwaert E, Van Biesen W, Raes A, Glorieux G, Van Bogaert V, Van Hoeck K, Coppens M, Roels S, Vande Walle J, Eloot S (2018) Concentrations of representative uraemic toxins in a healthy versus non-dialysis chronic kidney disease paediatric population. Nephrol Dial Transplant 33:978–986. https://doi.org/10.1093/ndt/gfx224
Bellemans M, De Maeyer M (2005) Maten en gewichten: handleiding voor een gestandardiseerde kwantificering van voedingsmiddelen. Second edition. edn, Brussels
Haycock GB, Schwartz GJ, Wisotsky DH (1978) Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr 93:62–66. https://doi.org/10.1016/s0022-3476(78)80601-5
Gezondheidsraad H (2016) Voedingsaanbevelingen voor België - 2016. Brussels
Krishnamurthy VM, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, Greene T, Beddhu S (2012) High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int 81:300–306. https://doi.org/10.1038/ki.2011.355
Sabatino A, Regolisti G, Gandolfini I, Delsante M, Fani F, Gregorini MC, Fiaccadori E (2017) Diet and enteral nutrition in patients with chronic kidney disease not on dialysis: a review focusing on fat, fiber and protein intake. J Nephrol 30:743–754. https://doi.org/10.1007/s40620-017-0435-5
Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G (2002) Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr 12:17–31
Stephen AM, Champ MM, Cloran SJ, Fleith M, van Lieshout L, Mejborn H, Burley VJ (2017) Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutr Res Rev 30:149–190. https://doi.org/10.1017/s095442241700004x
Kranz S, Brauchla M, Slavin JL, Miller KB (2012) What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv Nutr 3:47–53. https://doi.org/10.3945/an.111.001362
Chauveau P, Aparicio M, Bellizzi V, Campbell K, Hong X, Johansson L, Kolko A, Molina P, Sezer S, Wanner C, Ter Wee PM, Teta D, Fouque D, Carrero JJ (2018) Mediterranean diet as the diet of choice for patients with chronic kidney disease. Nephrol Dial Transplant 33:725–735. https://doi.org/10.1093/ndt/gfx085
Bres E, Koppe L (2019) Is there still a place for prebiotics in chronic kidney disease? Nephrol Dial Transplant 34:1812–1816. https://doi.org/10.1093/ndt/gfz124
McFarlane C, Ramos CI, Johnson DW, Campbell KL (2018) Prebiotic, probiotic, and synbiotic supplementation in chronic kidney disease: a systematic review and meta-analysis. J Ren Nutr 29:209–220. https://doi.org/10.1053/j.jrn.2018.08.008
Wu M, Cai X, Lin J, Zhang X, Scott EM, Li X (2018) Association between fibre intake and indoxyl sulphate/p-cresyl sulphate in patients with chronic kidney disease: meta-analysis and systematic review of experimental studies. Clin Nutr 38:2016–2022. https://doi.org/10.1016/j.clnu.2018.09.015
Pisano A, D'Arrigo G, Coppolino G, Bolignano D (2018) Biotic supplements for renal patients: a systematic review and meta-analysis. Nutrients 10:1224. https://doi.org/10.3390/nu10091224
Ramos CI, Armani RG, Canziani MEF, Dalboni MA, Dolenga CJR, Nakao LS, Campbell KL, Cuppari L (2018) Effect of prebiotic (fructooligosaccharide) on uremic toxins of chronic kidney disease patients: a randomized controlled trial. Nephrol Dial Transplant 34:1876–1884. https://doi.org/10.1093/ndt/gfy171
Lau WL, Savoj J, Nakata MB, Vaziri ND (2018) Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci (Lond) 132:509–522. https://doi.org/10.1042/cs20171107
Borges NA, Mafra D (2019) Chapter 36 - the gut microbiome in chronic kidney disease. In: Faintuch J, Faintuch S (eds) Microbiome and metabolome in diagnosis, therapy, and other strategic applications. Academic Press, pp 349-356. https://doi.org/10.1016/B978-0-12-815249-2.00036-1
Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, Pascual J, Ley RE, Spector TD, Bell JT, Menni C (2015) Gut-microbiota-metabolite axis in early renal function decline. PLoS One 10:e0134311. https://doi.org/10.1371/journal.pone.0134311
Acknowledgements
The authors wish to thank Sofie Vermeiren, Els Holvoet, Sophie Lobbestael, Tom Mertens, Maria Van Landschoot, An Desloovere, Sofie Eerens, Kimi Lambregts, Katrien Wellens, Jarrik Fransen, Imelda Hamels, Ariadne van Hulle, Katrien Van der Vaerent and Julia Versavau for their assistance.
Funding
This study was funded by the Agency for Innovation by Science and Technology (IWT), from the ‘Applied Biomedical Research with a Primary Societal Goal’ (TBM) program in Flanders (Belgium): UToPaed project, grant number IWT-TBM 150195. J.V.W. received lecture fees from Vitaflo and is member of the European Society for Paediatric Nephrology nutritional task force (with Vitaflo grant).
Author information
Authors and Affiliations
Contributions
Conceptualisation: Amina El Amouri, Evelien Snauwaert, Sunny Eloot, Ann Raes, Wim Van Biesen; methodology: Amina El Amouri, Evelien Snauwaert, Griet Glorieux, Sunny Eloot, Ann Raes; formal analysis and investigation: Amina El Amouri, Aurélie Foulon, Charlotte Vande Moortel, Evelien Snauwaert, Maria Van Dyck, Koen Van Hoeck, Nathalie Godefroid, Sunny Eloot; writing—original draft preparation: Amina El Amouri; writing—review and editing: Amina El Amouri, Evelien Snauwaert, Sunny Eloot, Ann Raes, Johan Vande Walle, Griet Glorieux, Wim Van Biesen; funding acquisition: Sunny Eloot, Ann Raes; resources: Wim Van Biesen, Griet Glorieux; supervision: Evelien Snauwaert, Sunny Eloot, Ann Raes.
Corresponding author
Ethics declarations
Conflict of interest
The other authors declare that they have no conflict of interest.
Ethics approval
Approval was obtained from the ethics committee at each participating centre.
Consent to participate
Written informed consent was obtained from the parents and children above the age of 12 years.
Consent for publication
Not applicable
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PPTX 68 kb)
Rights and permissions
About this article
Cite this article
El Amouri, A., Snauwaert, E., Foulon, A. et al. Dietary fibre intake is low in paediatric chronic kidney disease patients but its impact on levels of gut-derived uraemic toxins remains uncertain. Pediatr Nephrol 36, 1589–1595 (2021). https://doi.org/10.1007/s00467-020-04840-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-020-04840-9