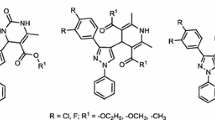
Abstract
In an effort to discover new imidazolyl nucleus belonging to the family of N-fused heterocyclic compounds which display broad spectrum of biological applications, a series of novel imidazolyl pyrazolopyridines 3a,b–7a,b and imidazolyl pyrazoloquinoxaline 8a–d were synthesized. The structures of all the synthesized compounds were confirmed using spectroscopic data and elemental analyses. The compounds were synthesized using conventional heating besides the environmentally friendly benign techniques and reagents as microwave technique and catalyst under solvent–free conditions and short reaction times by anomeric-based oxidation (ABO) to the products in the final step of the synthetic pathway. Further all the synthesized bioactive molecules are tested for their biological potency: in vitro antimicrobial activity using a disc diffusion technique was performed against various Gram-positive and Gram-negative bacteria as well as fungal strains using Chloramophenicol and Fluconazole as positive controls. Free radical scavenging activity has been investigated using the DPPH scavenging methods. Interestingly, most of the synthesized compounds exhibited good to excellent antibacterial activities against most of bacterial strains and showed the highest antioxidant activity.









Similar content being viewed by others
References
A. Kleeman, J. Engel, B. Kutscher, D. Reichert, Pharmaceutical Substances: Syntheses, Patents, Applications of the Most Relevant APIs, 3rd edn. (Thieme Medical, New York, NY, USA, 1999).
L.L. De, Curr. Med. Chem. 13(1), 1 (2006)
W.C. Yang, J. Li, J. Li, Q. Chen, G.F. Yang, Bioorg. Med. Chem. Lett. 22, 1455 (2012)
W. Xue-Quan, L. Lan-Xiang, Y. Xiao-Dong, Eur. J. Med. Chem. 62, 111 (2013). https://doi.org/10.1016/j.ejmech.2012.12.040
N.C. Desai, A.S. Maheta, K.M. Rajpara, V.V. Joshi, H.V. Vaghani, H.M. Satodiya, J. Saudi. Chem. Soc. 18, 963 (2011)
H.M. Alkahtani, A.Y. Abbas, S. Wang, Bioorg. Med. Chem. Lett. 22, 1317 (2012)
G.K. Sharma, S. Kumar, D. Pathak, Der. Pharm. Lett. 2, 22 (2010)
J. Pandey, V.K. Tiwari, S.S. Verma, V. Chaturvedi, S. Bhatnagar, S. Sinha, A.N. Gaikwad, R.P. Tripathi, Eur. J. Med. Chem. 44, 3350 (2009)
D. Zampieri, M.G. Mamolo, E. Laurini, G. Scialino, L. Banfi, E. Vio, Bioorg. Med. Chem. 16, 4516 (2008)
U. Ucucu, N.G. Karaburun, I. Isikdag, II Farmaco. 56, 285 (2001)
S. Kankala, R.K. Kankala, G. Prasad, N. Thota, S. Nerella, M.R. Gangula, H. Guguloth, M. Kagga, R. Vadde, C.S. Vasam, Bioorg. Med. Chem. Lett. 23, 1306 (2013)
P. Zhan, X. Liu, J. Zhu, Z. Fang, L. Zhenyu, C. Pannecouque, E.D. Clercq, Bioorg. Med. Chem. 17, 5775 (2009)
B.J. Ullas, P.G. Chandrashekar, R. Suhas, K.P. Rakesh, P. Avinash, D.C. Gowda, J. Chem. Appl. Biochem. 2, 1 (2015)
H.R.T. Ivan, H.R.A. Ali, M.A. Ahmed, F. Mohammed, J. Saudi. Chem. Soc. 20, S509 (2016)
F. Hadizadeh, H. Hosseinzadeh, V.S. Motamed-Shariaty, M. Seifi, S.H. Kazemi, Iran. J. Pharm. Res. (IJPR). 7, 29 (2008)
M. Gaba, C. Mohan, Med. Chem. Res. 25, 173 (2016). https://doi.org/10.1007/s00044-015-1495-5
S. Johny, R. Ivan, B. Jan, Synthesis 16, 2760 (2004)
E.J. Noga, G.T. Barthalmus, M.K. Mitchell, Cell. Biol. Int. 10, 239 (1986)
M.S. Subas, M.L.D. Kristi, L.M. Martha, R. Bryso, S. Andrei, J.R. Robert, A.K. David, C. Hengmiao, L. Jin, H.J. Burton, Bioorg. Med. Chem. Lett. 16, 288 (2006)
A.R. Katritzky, M. Wang, S. Zhang, M.V. Voronkov, J. Org. Chem. 66, 6787 (2001)
M. Li, B.X. Zhao, Eur. J. Med. Chem. 85, 311 (2014)
A.A. Nehad, M.S. Nermien, M.M. Ashraf, M.A. Mohamed, Monatsh. Chem. 138, 715 (2007)
E. Palaska, M. Ayemir, T. Uzbay, D. Erol, Eur. J. Med. Chem. 36(6), 539 (2001)
G.M. Nitulescu, G. Nedelcu, A. Buzescu, O.T. Olaru, Bulg. Chem. Commun. 48(1), 55 (2016)
A. Ranjana, K. Suresh, Beilstein. J. Org. Chem. 25(14), 203 (2018). https://doi.org/10.3762/bjoc.14.15
C.B. Vicebtini, C. Romangnoli, E. Andreotti, D. Mares, J. Agric. Food. Chem. 55, 10331 (2007)
V. K. Sundaravel, M. Shanmugam, P. Subbu, New. J. Chem. 51(1), (2019).
A. Anam, A. Abad, A. Mohd, Shamsuzzaman. New. J. Chem. 41, 16 (2017)
M. Soliman, J. Heterocyclic. Chem. 48, 592 (2011)
K. Srivani, E. Laxminarayana, M. Ramchander, M. Thirumala Chary, Indian. J. Heterocy. Chem. 29(3), 233 (2019).
A.S. Shawali, M.M. Zayed, T.A. Farghaly, J. Heterocyclic. Chem. 42(2), 185 (2005). https://doi.org/10.1002/jhet.5570420202
A.M. Mohamed, L. Salah, A.E.S. Mohammed, F.L. Farida, A. Thorleif, Curr. Opin. Green Sustain. Chem. 2, 71 (2012)
R. A. l. Aisha, S. A. Hebat-Allah, Molecules. 20(11), 19805 (2015). doi: https://doi.org/10.3390/molecules 201119655.
R. Ferraz, Biochem. Pharmacol. 03(01), (2014) DOI: https://doi.org/10.4172/2167-0501.1000e152.
M.M. Abbasi, S.M. El-Kousy, Y.E. El-Moghazy, S. El-Kafrawy, Int. J. Chem. 15(2), 77 (2005)
S.S. Ahmad, M.Z. Mohie, A.F. Thoraya, Chem. Inform. 36, 33 (2005). https://doi.org/10.1002/chin.200533176
M.A. Ortega, M.E. Montoya, B. Zarranz, A. Jaso, I. Aldana, S. Leclerc, L. Meijer, A. Monge, Bioorg. Med. Chem. 10(7), 2177 (2002). https://doi.org/10.1016/S0968-0896(02)00069-X
A.A. Ameen, Am. J. Org. Chem. 5(1), 14 (2015). https://doi.org/10.5923/j.ajoc.20150501.03
H.F. Rizk, M.A. El-Badawi, S.A. Ibrahim, M.A. El-Borai, Arab. J. Chem. 4, 37 (2011)
H.F. Rizk, S.A. Ibrahim, M.A. El-Borai, Dyes. Pigm. 112, 86 (2015)
M.A. Zolfigol, M. Yarie, Appl. Organometal. Chem. 31, e3598 (2017). https://doi.org/10.1002/aoc.3598S
F. Karimi, M. Yarie, M.A. Zolfigol, RSC. Adv. 10, 25828 (2020)
M. Kiafar, M. A. Zolfigol, M. Yarie, A (A). Taherpour, RSC. Adv., 6, 107295 (2016).
M. Yarie, Iran. J. Catal. 10(1), 79 (2020)
M. Yarie, Iran. J. Catal. 7(1), 85 (2017)
J. Afsar, M.A. Zolfigol, A. Khazaei, M. Zarei, Y. Gu, D.A. Alonso, A. Khoshnood, Mol. Catal. 482, 110666 (2020)
M.A. El-Borai, H.F. Rizk, S.A. Ibrahim, H.F. El-Sayed, J. Heterocycl. Chem. 54, 1031 (2017)
M.A. El-Borai, H.F. Rizk, S.A. Ibrahim, A.K. Fares, J. Heterocycl. Chem. 56, 2787 (2019)
H.F. Rizk, M.A. El-Badawi, S.A. Ibrahim, M.A. El-Borai, Chin. Chem. 29, 1451 (2011)
M. A. El-Borai, H. F. Rizk, D. M. Beltagy, I.Y El-Deeb, Eur. J. Med. Chem., 66, 415 (2013).
P. P. S. G. Divyaraj, N. P. Nayak, B. Manjunath, H. Hemant. J. Chin. Chem. Soc. 1(2020). DOI: https://doi.org/10.1002/jccs.201900388.
E.G. Fatma, A.M.A. Alaa, A. Omer, Bioorg. Med. Chem. 12, 1845 (2004). https://doi.org/10.1016/j.bmc.2004.01.040
P. Sukanya. R. R. C Venkata, J. chem. sci. 7(2), 105 (2017).
N. Harikrishna, M.I. Arun, K. Ananda, O. Abdulrahman, F. Hoong-Kun, RSC. Adv. 5, 43648 (2015)
M.A. El-Borai, K.A. Mohamed, H.F. Rizk, M.A. Faten, Curr. Org. Synth. 15, 275 (2017)
H.F. Rizk, M.A. El-Borai, A. Ragab, S.A.J. Ibrahim, Iran Chem. Soc. 17, 2493 (2020). https://doi.org/10.1007/s13738-020-01944-9
I. Kurniawati, A. Adam, Egypt. J. Aquat. Res. 42(4), 405 (2016). https://doi.org/10.1016/j.ejar.2016.10.005
A. Ardestani, R. Yazdanparast, Food. Chem. 104, 21 (2007)
http://dx.doi.org/https://doi.org/10.1016/j.foodchem.2006.10.066.
W.B. Williams, M.E. Cuvelier, C. Berset, Lebensm. Wiss. Technol. 28, 25 (1995)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ibrahim, S.A., Rizk, H.F., El-Borai, M.A. et al. Green routes for the synthesis of new pyrazole bearing biologically active imidiazolyl, pyridine and quinoxaline derivatives as promising antimicrobial and antioxidant agents. J IRAN CHEM SOC 18, 1391–1404 (2021). https://doi.org/10.1007/s13738-020-02119-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02119-2