Abstract
Scars are the normal outcome of wound repair and involve a co-ordinated inflammatory and fibrotic process. When a scar does not resolve, uncontrolled chronic inflammation can persist and elicits excessive scarring that leads to a range of abnormal phenotypes such as hypertrophic and keloid scars. These pathologies result in significant impairment of quality of life over a long period of time. Existing treatment options are generally unsatisfactory, and there is mounting interest in innovative cell-based therapies. Despite the interest in mesenchymal stem cells (MSCs), there is yet to be a human clinical trial that investigates the potential of MSCs in treating abnormal scarring. A synthesis of existing evidence of animal studies may therefore provide insight into the barriers to human application. The aim of this PRISMA systematic review was to evaluate the effectiveness of MSC transplantation in the treatment of hypertrophic and keloid scars in in vivo models. A total of 11 case-control studies were identified that treated a total of 156 subjects with MSCs or MSC-conditioned media. Ten studies assessed hypertrophic scars, and one looked at keloid scars. All studies evaluated scars in terms of macroscopic and histological appearances and most incorporated immunohistochemistry. The included studies all found improvements in the above outcomes with MSC or MSC-conditioned media without complications. The studies reviewed support a role for MSC therapy in treating scars that needs further exploration. The transferability of these findings to humans is limited by factors such as the reliability and validity of the disease model, the need to identify the optimal MSC cell source, and the outcome measures employed.
Similar content being viewed by others
Introduction
Wounds to the skin are caused by mechanical, thermal, and chemical trauma. Scars (or cicatrix) are the normal outcome of wound repair and involve a co-ordinated inflammatory and fibrotic process. Eventually, the scars remodel and become soft, flat, pale, and unobtrusive. When a scar does not resolve, persistent chronic inflammation can cause excessive scarring that lead to a range of abnormal phenotypes which clinically manifest as hypertrophic and keloid scars.
Hypertrophic scars affect nearly one in five people who suffer from burns and the risk of scarring increases with the time taken to heal (Chipp et al. 2017). They can also occur following incisional closure, a standard part of surgical procedures. Typically appearing within 2 months of injury, the disease process can be protracted and therefore carries significant societal and financial cost over a long period of time (Gangemi et al. 2008). Keloid scars impact tens of millions of people worldwide, and there is strong evidence of a significant genetic predisposition (Bayat et al. 2003; Santos-Cortez et al. 2017). In contrast to hypertrophic scars, keloid scars can appear much later post-injury and are characterised by extension beyond the original area of the trauma. Ultimately, hypertrophic and keloid scars result in significant impairment of quality of life (Bock et al. 2006). In addition to cosmetic consequences, these abnormal scars can have functional implications including restricted mobility, pain, and pruritus (Bijlard et al. 2017; Lee et al. 2004).
Excess scarring may persist and often recurs after multiple interventions (Darzi et al. 1992; Gauglitz et al. 2011). Most patients suffer from neuropathic pain and pruritus, and the mainstay of treatment is conservative therapy (Argirova et al. 2006). However, existing treatment options are generally unsatisfactory for patients and doctors alike. In particular, surgery, which is mainly focused on scar excision, has a very high recurrence rate whether used alone or in combination with depot steroids (Berman et al. 2009; Furtado et al. 2012; Wilson 2013). Strategies aimed at scar growth suppression include topical treatments such as retinoic acid, imiquimod, and corticosteroid injections (Jacob et al. 2003; Janssen De Limpens 1980). These remedies tend to demonstrate only short-term efficacy (Berman et al. 2009; Cação et al. 2009). Repeated steroid injections are nevertheless efficacious. Pressure therapy and silicone gel cream or sheets stand out as clinically useful and widely used measures both therapeutically and preventatively (Ai et al. 2017; Kim et al. 2014). Modalities such as radiotherapy, cryotherapy, and lasers have either high failure rates, and/ or carry risk of adverse events, not to mention high cost (Manuskiatti and Fitzpatrick 2002; Puri and Talwar 2009; Song et al. 2014; Steinstraesser et al. 2011). Therefore, there is mounting interest in innovative methods to treat hypertrophic and keloid scars. Emerging studies have therefore taken a different approach and focussed on cell-based therapies such as mesenchymal stem cells (MSCs) (Fung et al. 2017).
MSCs are adult multipotent stromal cells that can be readily harvested from various sites such as bone marrow, adipose, and umbilical tissue (Baksh et al. 2007; Khan et al. 2008). MSCs can be expanded ex vivo and cultured under specific conditions to promote particular cellular effects. Due to their low immunogenicity, MSCs are frequently transplanted allogeneically for the treatment of inflammatory conditions (Kabat et al. 2020). MSCs exert their anti-inflammatory and anti-fibrotic paracrine effects via the chemokines and microvesicles that they secrete (Badiavas et al. 2003; Horwitz and Dominici 2008; Rani et al. 2015). Excessive scarring involves undesired inflammation that results in deposition of immature extracellular matrix (ECM) by fibroblasts and myofibroblasts (Barallobre-Barreiro et al. 2019). Whilst tissue native MSCs play a key role in potentiating this process, there is evidence to suggest that transplanted MSCs are instead able to attenuate inflammation and promote a return to homeostasis (Chen et al. 2009; Ren et al. 2008). MSCs may achieve this by mediating macrophage class switch from a proinflammatory M1 to anti-inflammatory M2 phenotype (Cho et al. 2014). MSCs also have the potential to negatively modulate ECM deposition, possibly via promoting a T-cell response that results in the downregulation of TGF-β1, a key regulator of collagen synthesis (Huang et al. 2015; Spiekman et al. 2014).
Despite the interest in MSCs, there is yet to be a human clinical trial that investigates the potential of MSCs in treating excessive scarring. A synthesis of existing evidence of animal studies will therefore provide insight into the barriers to human application. The aim of this systematic review was to evaluate the effectiveness of MSC transplantation in the treatment of hypertrophic and keloid scars in in vivo models.
Materials and methods
A literature search was performed using PubMed, Web of Science, and Cochrane Database from conception to May 2020. The following search terms were used: ((((((((MSC) OR Mesenchymal Stem Cell) OR Mesenchymal Stromal Cell) OR Multipotent Stem Cell) OR Multipotent Stromal Cell) OR Stem Cell)) AND ((Keloid) OR Hypertrophic)) AND Scar.
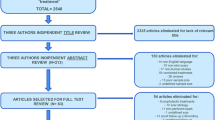
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and included case control, cohort studies, case series, and randomised controlled trials (Moher et al. 2009). A total of 1098 studies were subjected to the inclusion/exclusion criteria, yielding a final 11 studies for qualitative analysis (Fig. 1). Studies that evaluated MSC or MSC-conditioned media transplantation as therapies were included. Studies that assessed in vivo models were included. Studies of all design were included. Literature reviews, systematic reviews, and case reports were excluded but were reverse-reference searched to maximise yield. Studies with only in vitro experiments were excluded. All included studies were published in the English language, and all unpublished, inaccessible, and retracted literature were excluded. CB and KT carried out the search independently. Risk of bias was assessed by AH and JS using the SYRCLE RoB tool (Table 1; Fig. 2) (Hooijmans et al. 2014).
Results
A total of 11 studies were identified (Tables 2, 3, and 4) (Domergue et al. 2016; Foubert et al. 2017; Hu et al. 2019, 2020; Li et al. 2016; Liu et al. 2018, 2014; Yates et al. 2017; Yates et al. 2017; Zhang et al. 2015). A total of 156 subjects were treated with MSCs or MSC-conditioned media. There were no significant complications reported in any of the studies. Ten studies assessed the effectiveness of MSCs or MSC-conditioned media in treating hypertrophic scars and one in keloid scars. All studies were case control studies.
MSC isolation and characterisation
Six studies used bone marrow MSCs: two of murine origin (Hu et al. 2019, 2020), two of human origin (Yates et al. 2017; Yates et al. 2017), one of rabbit origin (Liu et al. 2014), and one study included both human and rabbit origin MSCs (Liu et al. 2014). Five of the studies that employed bone marrow MSCs harvested cells by needle aspiration from either the tibia, femur or posterior iliac crest (Hu et al. 2019, 2020; Liu et al. 2014; Liu et al. 2014; Yates et al. 2017). One study, by Yates et al. (Yates et al. 2017) used bone marrow MSCs derived from an immortalised cell line. Five studies utilised adipose MSCs: three of human (Domergue et al. 2016; Li et al. 2016; Liu et al. 2018), one of porcine (Foubert et al. 2017) and one of rabbit origin (Zhang et al. 2015). Three studies experimented MSCs from inguinal fat pad or redundant tissue from surgical operations (Foubert et al. 2017; Liu et al. 2018; Zhang et al. 2015). Domergue et al. (2016) extracted MSCs by dermolipectomy and Li et al. (2016) by liposuction. Whilst all the studies applied flow cytometry to characterise MSCs, only four satisfied the International Society for Cellular Therapy (ISCT) criteria for defining MSCs by also performing tri-lineage differentiation (Dominici et al. 2006; Hu et al. 2019, 2020; Yates et al. 2017; Yates et al. 2017). Five studies performed bi-lineage differentiation only (Li et al. 2016; Liu et al. 2018, 2014; Liu S. et al. 2014; Zhang et al. 2015).
MSC treatment and delivery
Most studies passaged MSCs at least three times. Only two studies used MSCs from earlier passage; Domergue et al. (2016) used passage one, and Foubert et al. (2017) did not passage the cells at all. Interestingly, two studies harvested MSCs beyond the eighth passage (Hu et al. 2019, 2020). Studies transplanted varying concentrations of MSCs but at similar volumes of around 200 μl. Two studies administered 1000 μl (Li et al. 2016; Liu et al. 2014), whilst three studies dispensed less than 100 μl of MSCs (Domergue et al. 2016; Liu. et al. 2018; Yates et al. 2017). Yates et al. (Yates et al. 2017) did not specify the quantity given. The routes of MSC administration were highly variable. Eight of the eleven studies delivered MSCs or MSC-conditioned media by subcutaneous injection. Of these, four studies specified further; two injected four points of the wound (Domergue et al. 2016; Liu et al. 2018), one injected into the centre of each wound (Zhang et al. 2015), and the fourth delivered MSCs by circumferential intradermal injection into each wound (Liu et al. 2014). Of the remaining three studies, one study delivered MSCs onto the wound via an aerosol (Foubert et al. 2017), one applied the MSCs to fill the wound defect (Yates et al. 2017), and one injected MSCs intra-arterially (Liu et al. 2014). Five of the eleven studies utilised MSC-conditioned media (Hu et al. 2019, 2020; Li et al. 2016; Liu et al. 2018; Zhang et al. 2015). Two studies used chemokine receptor 3 (CXCR3) knockout mice, which are known to scar excessively when wounded (Yates et al. 2017; Yates et al. 2017). Four studies employed internal controls by injecting MSCs on the contralateral side of the animal subject (Foubert et al. 2017; Hu et al. 2019; Yates et al. 2017; Zhang et al. 2015). Five studies utilised Dulbecco’s modified Eagle media (DMEM) (Hu et al. 2019, 2020; Li et al. 2016; Liu et al. 2018; Zhang et al. 2015), and three applied phosphate buffer solution (PBS) as controls (Domergue et al. 2016; Liu et al. 2014; Liu et al. 2014). Other control groups comprised lactated Ringer’s solution (LR), hyaluronic acid (HA), and no treatment as a control (Foubert et al. 2017; Yates et al. 2017; Yates et al. 2017). The majority of studies followed up wound progression for at least 28 days.
Disease model
Eight studies evaluated the effectiveness of MSCs in preventing hypertrophic scar formation (Table 3), and three studies examined MSC therapy on formed scars. In the latter, one study assessed keloid scars and included four subjects. Six studies assessed murine, four used rabbit, and one utilised a porcine subject. All the induced wounds were full dermal-thickness but varied in size and location, with the majority being circular punch wounds inflicted on the dorsum of murine subjects. Four studies inflicted full-thickness punch wounds on the ears of rabbit subjects (Hu et al. 2019; Liu et al. 2014; Liu et al. 2014; Zhang et al. 2015). Three studies (Table 4) created full-thickness skin wounds on human skin samples which were then xenografted onto murine subjects (Domergue et al. 2016; Hu et al. 2020; Liu et al. 2018).
Treatment outcomes and complications
All studies assessed wounds in terms of macroscopic appearance and histology with most including immunohistochemistry. No complications were reported by any of the studies. Gross appearance was evaluated in all studies using high-resolution photography, and all studies reported positive improvements in various measured parameters in the MSC-treated group compared with controls. Eight studies described reduced scar hypertrophy in the MSC-treated group compared with controls (Domergue et al. 2016; Foubert et al. 2017; Hu et al. 2019, 2020; Li et al. 2016; Yates et al. 2017; Yates et al. 2017; Zhang et al. 2015). Two studies reported that MSC-treated subjects attenuated hypertrophic scar formation (Liu et al. 2014; Liu et al. 2014). One study evaluated keloid size and found greater scar shrinkage following treatment (Liu et al. 2018). Several studies assessed collagen characteristics using assays of collagen gel contraction (Hu et al. 2019), collagen deposition (Foubert et al. 2017; Hu et al. 2020), and collagen content (Domergue et al. 2016). All studies reported reduced collagen deposition and reduced collagen contracture in the MSC-treated group compared with controls. Two studies assessed fibroblast apoptosis. Hu et al. (2020) found increased fibroblast apoptosis by staining for caspase-7. Liu et al. (2018) measured the presence of phosphatidylserine in the outer layer of the phospholipid bilayer as a surrogate marker of apoptosis and found no change in the MSC-treated group compared with control. Another study used TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labelling) staining to assess MSC apoptosis and found that a significant proportion of MSCs underwent apoptosis after administration onto a wound (Liu et al. 2014). Three of the eleven studies assessed scar thickness, with two using digital planimetry (Foubert et al. 2017; Liu et al. 2014) and one using ultrasonography (Zhang et al. 2015). All studies reported reduced scar tissue height and hardness. Yates et al. (2017), by staining caspase-3 with a fluorescent probe, found reduced caspase-3, suggesting improved fibroblast survival following MSC co-transplantation.
Discussion
Although the outcomes reported in this review generally favour MSC transplantation in treating excessive scarring and did not report complications, it is difficult to draw reliable conclusions due to the heterogeneity of the studies. This arises from various aspects; there was significant variability in the cell source, cell treatment, method of delivery, and the disease model used to assess efficacy. Most studies demonstrated moderate to high overall risk of bias as they were aiming to different and more specific questions relevant to MSC use. Nevertheless, this systematic review provides a useful summary and helps inform future study design.
The properties of MSCs can vary according to the cell source. Consistent with the existing literature, most of our studies examined adipose MSCs (AMSCs) and bone marrow MSCs (BMMSCs) (Kabat et al. 2020). Both of these cell sources have their relative advantages for use in treating scars. AMSCs offer a greater capacity to proliferate ex vivo compared with other cell sources (Peng et al. 2008) and therefore may be suitable for large scale off-the-shelf preparations at greater cost-effectiveness. They may also be more abundant, less invasive to harvest, and are often available as medical waste in many cosmetic surgery procedures. BMMSCs may represent a less heterogenous cell population (Liu et al. 2013) but exhibit senescence at earlier passage (Burrow et al. 2017). An important consideration is that the anti-inflammatory properties of MSCs could differ by cell source. Particular studies suggest that AMSCs may be superior in promoting an M1 to M2 phenotype transition in macrophages that favour resolution of inflammation (Heo et al. 2019). This is relevant as macrophages are a key mediator of the pathogenic process of excessive scarring (Feng et al. 2019; Hesketh et al. 2017). In addition, certain MSCs demonstrate a greater ability to engraft onto lesions and can therefore produce more sustained effects (Burk et al. 2016). One study in this review compared human and rabbit cell sources and found both cell sources to be equally efficacious (Liu et al. 2014). Harvesting MSCs from animals rather than humans may be more convenient but the immunogenic consequences of xenogeneic transplantation with human recipients are yet to be thoroughly investigated. Although heterogeneity of MSC origin and culture condition among the included studies may affect the reliability of conclusions drawn from them, it is reassuring that positive effects were observed across multiple cell sources. This indicates that MSCs regardless of origin have the potential to treat hypertrophic and keloid scars. Future studies should aim to identify the best cell source for treating excessive scarring.
Significant heterogeneity was also observed between the studies in terms of culture conditions and treatment delivery methods. The literature suggests that pre-conditioning MSCs with inflammatory cytokines may serve to promote an anti-inflammatory MSC phenotype (Saldaña et al. 2019). Similarly, following co-culture with fibroblasts, a cell type prevalent in inflamed scars, MSCs express greater levels of anti-inflammatory cytokines (Suzuki et al. 2017). This suggests that treating MSCs in conditions reflective of the scar environment might potentiate their effectiveness when used in transplantation. Conversely, serum-free culture conditions appear to enhance the anti-fibrotic properties of MSCs in vivo (Yoshida et al. 2018). This may represent a potential challenge as the optimal culture protocol should promote an anti-fibrotic response without compromising the anti-inflammatory properties of MSCs. One way of circumventing this could be to stimulate MSCs under a particular set of culture conditions, and then harvesting the conditioned media that contains bioactive extracellular vesicles (EVs). The MSCs can then be resuspended and grown under a different set of culture conditions to promote secretion of different bioactive substances. Indeed, several studies in our review showed that a cell-free treatment using MSC-conditioned media can be effective (Hu et al. 2019, 2020; Li et al. 2016; Zhang et al. 2015).
On the other hand, it is difficult to identify the best MSC delivery method. MSCs injected into the circulation appear to engraft well into wounds (Deng et al. 2005), but carry a risk of interacting with cytokines and drugs present in the serum, which may alter MSC function (Javorkova et al. 2018). In contrast, MSCs injected directly into a lesion of interest could delocalise rapidly (Burk et al. 2016) and therefore still have the potential to exert off-site effects (Devine et al. 2003). Although there were no complications reported in any of the studies in this review, several factors have the potential to influence MSC biodistribution and therefore clinical efficacy following administration. It has been reported that pulmonary complications relating to IV administration of MSCs could be dependent on the cell suspension formulation (Deak et al. 2010). Other studies suggest that following initial localisation in the lungs following systemic administration, MSCs can home to areas of inflammation (Rustad and Gurtner 2012). Although useful in cases of isolated skin pathology, undesired offsite effects may be observed in cases of other underlying systemic inflammation (Gholamrezanezhad et al. 2011). There is also evidence to show that the migration and proliferation of MSCs at skin wounds can be a function of MSC expression of adhesion molecules including junction adhesion molecule A (JAM-A) (Wu et al. 2015). Likewise, chemokines such as CCR7 also appear to promote MSC migration to skin wounds (Sasaki et al. 2008). For the purposes of treating scars, it appears that local administration may be preferable, with recent studies demonstrating safety in animals via subcutaneous (Tappenbeck et al. 2019) and topical (Beyazyildiz et al. 2014) routes. Robust experiments that compare methods of MSC delivery in treating scars should address this ambiguity.
It remains uncertain whether interpretations drawn from animal models of excessive scarring can be transferred directly to inform treatment in humans. Most of the studies in this review assessed the effects of MSCs on the degree of hypertrophy during the scarring process. This probably does not replicate the human disease where patients typically present with a fully formed scar. Nevertheless, it may inform whether MSCs can be implemented at the time of injury (in high risk patients) or shortly after or in conjunction with surgical scar treatment as a means of preventing primary or recurrent hypertrophic or keloid scars. Genetic models of hypertrophic scarring may confer high reproducibility. There are existing gain-of-function models such as the Tight Skin 2 mouse which exhibit increased fibrosis following injury (Long et al. 2014), presumably due to increased collagen III alpha-1 expression (Long et al. 2015). Instead of a gain-of-function model, the two studies by Yates et al. (Yates et al. 2017; Yates et al. 2017) captured in this review utilised a previously validated knockdown model by targeting the CXCR3 gene (Yates et al. 2010). Whilst both methods may be informative for in vivo studies of hypertrophic scarring, they do not reflect the pattern of genetic predisposition in humans (Zhu et al. 2013), and the knock-down target does not correlate with known protective genetic variants (Sood et al. 2015). It is suggested that concomitantly xenografting human skin cells into the wound may improve the validity of the mouse burns model by promoting a more extensive scar phenotype (Ibrahim et al. 2014; Momtazi et al. 2013). However, this could be confounded by the immunogenic effects of xenografting skin onto an immunocompetent mouse (Racki et al. 2010). Nevertheless, the studies in this review that conducted xenografting of human skin into mouse defects did not observe graft rejection (Domergue et al. 2016; Hu et al. 2019; Liu et al. 2018).
Another issue relates to the time-course of scar pathogenesis. Most mouse models develop mature hypertrophic or keloid scarring within days to weeks after burn injury and weeks to months after incisional injury (Kim et al. 2018), unlike the longer time course of human disease. In humans, excessive scarring can occur after months (Gangemi et al. 2008), with biomolecular evidence of active disease at up to a year later (Van Der Veer et al. 2011). There is evidence in the literature to support the potential use of the Red Duroc porcine model, which develops scarring over months instead, and therefore better recapitulates the human process (Harunari et al. 2006; Zhu et al. 2003, 2004). We captured one study by Foubert et al. (2017) that was able to utilise this model in order to undertake an extended follow-up period of six months, when active scar growth was still observed. Whilst all of the studies demonstrated sustained benefit and did not report recurrence up to the end point of follow-up, keloid and hypertrophic scars are known in humans to recur after many months to years following successful treatment (Furtado et al. 2012). Therefore, the short lifespan of murine models may not permit sufficient longitude to assess whether the benefits of MSC therapy is sustained. Future studies of porcine models with long follow-up periods may facilitate this.
In order to fully exploit the beneficial effects of MSCs in treating scars, it is important to establish a dose-response relationship. The studies in this review varied significantly in the amount of MSC or MSC-conditioned media used, but all reported positive outcomes. Only one study examined the effects of varying the dose of MSC-conditioned media used and found a dose-response relationship (Li et al. 2016). It is unclear whether the same relationship may be observed in treatment with MSCs of varying concentration and there is evidence in models of ischaemic injury that higher doses of MSCs do not always confer greater therapeutic benefit (Yavagal et al. 2014). Therefore, a relevant future study might aim to determine the maximum tolerated dose (MTD) for MSCs in treating keloid and hypertrophic scars. The method of delivery might influence this, as appropriate dosage for intravenous injection may be derived from the weight of the subject, whereas intralesional delivery may require the volume of the scar of interest to be calculated. Digital planimetry, as employed by several studies here, may be a viable method of achieving this (Foubert et al. 2017; Liu et al. 2014). Ascertaining the MTD will also inform safe dosages that do not evoke adverse effects (Karussis et al. 2010). Six studies in this review treated scars with MSCs, four studies used conditioned media, and one compared the two. There has been an emerging body of evidence to support the use of conditioned media, which contains bioactive extracellular vesicles (EVs) that may be the active therapeutic ingredient of MSCs (Furuta et al. 2016). As a cell-free therapy, it is possible that EVs are less immunogenic and may therefore be more suitable for large-scale production from allogeneic sources (Monguió-Tortajada et al. 2017).
Outcome measures utilised by in vivo studies can limit their transferability to humans. Whilst reduction in scar size and improvement in histological appearance may reflect the cosmetic benefits of treatment, it is unclear how it affects scar symptoms. As pain and pruritis are the main symptoms of hypertrophic and keloid scars (Lee et al. 2004), functional assessments in animals may be crucial before undertaking human trials. For example, there are well-validated and quantifiable behavioural measures such as vocalisation that reflect pain in mice (Kurejova et al. 2010). Assessing the degree of physical activity such as time spent digging (Shepherd et al. 2018) could potentially reveal the functional implications of contractures resulting from scars, although this could be dependent on the position of the lesion. Aside from looking to reduce the amount of scarring, there is a range of symptoms that can be caused by excessive scarring, and so separate studies may be required to evaluate the differential benefits of MSC therapy and to determine a personalised approach according to the specific symptom.
Conclusion
The present review suggests that mesenchymal stem cell (MSC) therapy can be an effective method of treating hypertrophic and keloid scars across a range of cell sources and animal models and does not cause significant complications. However, there is inadequate high-level evidence of in-human studies to support clinical efficacy in humans. There are several areas that need to be addressed before proceeding to human trials. This includes the identification of a reliable, reproducible, and validated animal model, and a standardised method of MSC delivery to allow a dose-response relationship to be established. The similar positive results observed to date with MSCs and MSC-conditioned media are encouraging and should be explored further by assessing the efficacy of MSC-derived extracellular vesicles, as this will carry significant implications for cost-effectiveness in treating humans at a population scale.
References
Wei AJ , Tao LJ , Duo PS , Li LY , Lin DS , Ming H, Bin P et al (2017) The effectiveness of pressure therapy (15–25 MmHg) for hypertrophic burn scars: a systematic review and meta-analysis. Scientific Reports 7
Argirova M, Hadjiski O, Victorova A et al (2006) Non-operative treatment of hypertrophic scars and keloids after burns in children. Ann Burns Fire Disasters 19(2):80–87
Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P et al (2003) Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 196(2):245–250
Baksh D, Yao R, Tuan RS et al (2007) Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25(6):1384–1392
Barallobre-Barreiro J, Woods E, Bell RE, Easton JA, Hobbs C, Eager M, Baig F, Ross AM, Mallipeddi R, Powell B, Soldin M, Mayr M, Shaw TJ et al (2019) Cartilage-like composition of keloid scar extracellular matrix suggests fibroblast mis-differentiation in disease. Matrix Biol Plus 4:100016
Bayat A, McGrouther DA, Ferguson MWJ et al (2003) Skin scarring. BMJ 326(7380):88–92
Berman B, Harrison-Balestra C, Perez OA, Viera M, Villa A, Zell D, Ramirez C et al (2009) Treatment of keloid scars post-shave excision with imiquimod 5% cream: a prospective, double-blind, placebo-controlled pilot study. J Drugs Dermatol 8(5):455–458
Emrullah B, Alpaslan PF, Özlem B, Rümeysa HE, Uʇur A, Necati DM, Aynur A, Figen K, Güngör S, Tuncay D et al (2014) Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int 2014
Bijlard E, Kouwenberg CAE, Timman R, Hovius SER, Busschbach JJV, Mureau MAM et al (2017) Burden of keloid disease: a cross-sectional health-related quality of life assessment. Acta Dermato-Venereologica 97(2):225–229
Bock O, Schmid-Ott G, Malewski P, Mrowietz U et al (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297(10):433–438
Burk J, Berner D, Brehm W, Hillmann A, Horstmeier C, Josten C, Paebst F, Rossi G, Schubert S, Ahrberg AB et al (2016) Long-term cell tracking following local injection of mesenchymal stromal cells in the equine model of induced tendon disease. Cell Transpl 25(12):2199–2211
Burrow K, Hoyland JA, Richardson SM et al (2017) Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro
Cação FM, Tanaka V, Messina MC, Lourenzo De et al (2009) Failure of imiquimod 5% cream to prevent recurrence of surgically excised trunk keloids. Dermatol Surg 35(4):629–633
Chen L, Tredget EE, Liu C, Wu Y et al (2009) Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS ONE 4(9)
Chipp E, Charles L, Thomas C, Whiting K, Moiemen N, Wilson Y et al (2017) A prospective study of time to healing and hypertrophic scarring in paediatric burns: every day counts. Burns and Trauma 5
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y et al (2014) Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 46(1):e70–e70
Darzi MA, Chowdri NA, Kaul SK, Khan M et al (1992) Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up study. Br J Plast Surg 45(5):374–379
Deak E, Rüster B, Keller L, Eckert K, Fichtner I, Seifried E, Henschler R et al (2010) Suspension medium influences interaction of mesenchymal stromal cells with endothelium and pulmonary toxicity after transplantation in mice. Cytotherapy 12(2): 260–64. https://pubmed.ncbi.nlm.nih.gov/19929457/ (October 11, 2020)
Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You S, Deng H, Murad F, Zhao RCH et al (2005) Engrafted bone marrow-derived Flk-1+ mesenchymal stem cells regenerate skin tissue. Tissue Eng 11(1–2):110–119
Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R et al (2003) Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 101(8):2999–3001
Domergue S, Bony C, Maumus M, Toupet K, Frouin E, Rigau V, Vozenin M-C, Magalon G, Jorgensen C, Noël D et al (2016) Comparison between stromal vascular fraction and adipose mesenchymal stem cells in remodeling hypertrophic scars ed Alexander V Ljubimov. PLOS ONE 11(5):e0156161
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy 8(4):315–317
Feng Y, Sun ZLL, Si YW, Jun JZ, Bin HL, Guo ZD, Yong Y, Shun Y, Ming LY, Feng L, Zhou XJ et al (2019) Direct and indirect roles of macrophages in hypertrophic scar formation. Frontiers in Physiology 10(AUG)
Foubert P, Zafra D, Liu M, Rajoria R, Gutierrez D, Tenenhaus M, Fraser JK et al (2017) Autologous adipose-derived regenerative cell therapy modulates development of hypertrophic scarring in a red duroc porcine model. Stem Cell Research and Therapy 8(1)
Fung M, Yuan Y, Atkins H, Shi Q, Bubela T et al (2017) Responsible translation of stem cell research: an assessment of clinical trial registration and publications. Stem Cell Rep 8(5):1190–1201
Furtado F, Hochman B, Ferreira LM et al (2012) Evaluating keloid recurrence after surgical excision with prospective longitudinal scar assessment scales. J Plast, Reconstr Aesthet Surg 65(7):e175–e181
Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M et al (2016) Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. STEM CELLS Transl Med 5(12):1620–1630
Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo M, Bollero D, Ganem J, Capocelli R, Cuccuru F, Cassano P, Risso D, Stella M et al (2008) Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 10(2):93–102
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG et al (2011) Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med 17(1–2): 113–25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3022978/
Gholamrezanezhad A, Mirpour S, Bagheri M, Mohamadnejad M, Alimoghaddam K, Abdolahzadeh L, Saghari M, Malekzadeh R et al (2011) In vivo tracking of 111in-oxine labeled mesenchymal stem cells following infusion in patients with advanced cirrhosis. Nucl Med Biol 38(7): 961–67. https://pubmed.ncbi.nlm.nih.gov/21810549/ (October 11, 2020)
Harunari N, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, Isik FF, Gibran NS, Engrav LH et al (2006) Histology of the thick scar on the female, red duroc pig: final similarities to human hypertrophic scar. Burns 32(6):669–677
Heo JS, Choi Y, Kim HO et al (2019) Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int 2019
Hesketh M, Sahin KB, West ZE, Murray RZ et al (2017) Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci 18(7)
Hooijmans CR, Rovers MM, Vries De, Rob BM, Leenaars M, Ritskes-Hoitinga M, Langendam MW et al (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14(1):43
Horwitz EM, Dominici M (2008) How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy 10(8):771–774
Hu CH, Tseng YW, Chiou CY, Lan KC, Chou CH, Tai CS, Da Huang H, Hu CW, Liao KH, Chuang SS, Yang JY, Lee OK et al (2019) Bone marrow concentrate-induced mesenchymal stem cell conditioned medium facilitates wound healing and prevents hypertrophic scar formation in a rabbit ear model. Stem Cell Res Ther 10(1):275
Hu CH, Tseng YW, Lee CW, Chiou CY, Chuang SS, Yang JY, Lee OK et al (2020) Combination of mesenchymal stem cell-conditioned medium and botulinum toxin type A for treating human hypertrophic scars. J Plast, Reconstr Aesthet Surg 73(3):516–527
Huang S, Wu Y, Gao D, Fu X et al (2015) Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice. Cytotherapy 17(7): 922–31. http://www.ncbi.nlm.nih.gov/pubmed/25939802
Ibrahim MM, Bond J, Bergeron A, Miller KJ, Ehanire T, Quiles C, Lorden ER, Medina MA, Fisher M, Klitzman B, Selim MA, Leong KW, Levinson H et al (2014) A novel immune competent murine hypertrophic scar contracture model: a tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen 22(6):755–764
Jacob SE, Berman B, Nassiri M, Vincek V et al (2003) Topical application of imiquimod 5% cream to keloids alters expression genes associated with apoptosis. In British Journal of Dermatology, Supplement. 62–65
Janssen De Limpens AMP (1980) The local treatment of hypertrophic scars and keloids with topical retinoic acid. Br J Dermatol 103(3):319–323
Javorkova E, Vackova J, Hajkova M, Hermankova B, Zajicova A, Holan V, Krulova M et al (2018) The effect of clinically relevant doses of immunosuppressive drugs on human mesenchymal stem cells. Biomed Pharmacother 97:402–411
Kabat M, Bobkov I, Kumar S, Grumet M et al (2020) Trends in mesenchymal stem cell clinical trials 2004–2018: is efficacy optimal in a narrow dose range? STEM CELLS Transl Med 9(1):17–27
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, Slavin S et al (2010) Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67(10):1187–1194
Khan WS, Tew SR, Adesida AB, Hardingham TE et al (2008) Human Infrapatellar fat pad-derived stem cells express the pericyte marker 3G5 and show enhanced chondrogenesis after expansion in fibroblast growth factor-2. Arthritis Res Ther 10(4):R74
Kim M, Kim H, Kang HW et al (2018) Comparative evaluations of hypertrophic scar formation in in vivo models. Lasers Surg Med 50(6):661–668
Kim SM, Choi JS, Lee JH, Kim YJ, Jun YJ et al (2014) Prevention of postsurgical scars: comparsion of efficacy and convenience between silicone gel sheet and topical silicone gel. J Korean Med Sci 29(Suppl 3):S249–S253
Kurejova M, Nattenmüller U, Hildebrandt U, Selvaraj D, Stösser S, Kuner R et al (2010) An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer pain and neuropathic pain. Mol Pain 6:18
Lee SS, Yosipovitch G, Chan YH, Goh CL et al (2004) Pruritus, pain, and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol 51(6):1002–1006
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, Yang X, He T, Guan H, Zheng Z, Han S, Dong M, Han J, Shi J, Hu D et al (2016) Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the P38/MAPK signaling pathway. Stem Cell Res Ther 7(1):102
Liu J, Ren J, Su L, Cheng S, Zhou J, Ye X, Dong Y, Sun S, Qi F, Liu Z, Pleat J, Zhai H, Zhu N et al (2018) Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 44(2):370–385
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, Yu C, Jin Y et al (2014) Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Investig Dermatol 134(10):2648–2657
Liu X, Wang Z, Wang R, Zhao F, Shi P, Jiang Y, Pang X et al (2013) Direct comparison of the potency of human mesenchymal stem cells derived from amnion tissue, bone marrow and adipose tissue at inducing dermal fibroblast responses to cutaneous wounds. Int J Mol Med 31(2):407–415
Liu YL, Liu WH, Sun J, Hou TJ, Liu YM, Liu HR, Luo YH, Zhao NN, Tang Y, Deng FM et al (2014) Mesenchymal stem cell-mediated suppression of hypertrophic scarring is P53 dependent in a rabbit ear model. Stem Cell Res Ther 5(6)
Long KB, Li Z, Burgwin CM, Choe SG, Martyanov V, Sassi-Gaha S, Earl JP, Eutsey RA, Ahmed A, Ehrlich GD, Artlett CM, Whitfield ML, Blankenhorn EP et al (2015) The Tsk2/+ mouse fibrotic phenotype is due to a gain-of-function mutation in the piiinp segment of the Col3a1 gene. J Investig Dermatol 135(3):718–727
Long KB, Artlett CM, Blankenhorn EP et al (2014) Tight skin 2 mice exhibit a novel time line of events leading to increased extracellular matrix deposition and dermal fibrosis. Matrix Biol 38:91–100
Manuskiatti W, Fitzpatrick RE (2002) Treatment response of keloidal and hypertrophic sternotomy scars: comparison among intralesional corticosteroid, 5-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol 138(9):1149–1155
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, Clark J, Clarke M, Cook D, D’Amico R, Deeks JJ, Devereaux PJ, Dickersin K, Egger M, Ernst E et al (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine 6(7)
Momtazi M, Kwan P, Ding J, Anderson CC, Honardoust D, Goekjian S, Tredget EE et al (2013) A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen 21(1):77–87
Monguió-Tortajada M, Roura S, Gálvez-Montón C, Pujal JM, Aran G, Sanjurjo L, Franquesa M, Sarrias MR, Bayes-Genis A, Borràs FE et al (2017) “Nanosized UCMSC-Derived Extracellular Vesicles but Not Conditioned Medium Exclusively Inhibit the Inflammatory Response of Stimulated T Cells: Implications for Nanomedicine. Theranostics 7(2): 270–84
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C et al (2008) Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 17(4):761–773
Puri N, Talwar A (2009) The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Sur 2(2):104
Racki WJ, Covassin L, Brehm M, Pino S, Ignotz R, Dunn R, Laning J, Graves SK, Rossini AA, Shultz LD, Greiner DL et al (2010) NOD-Scid IL2rγnull mouse model of human skin transplantation and allograft rejection. Transplant 89(5):527–536
Rani S, Ryan AE, Griffin MD, Ritter T et al (2015) Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther 23(5):812–823
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y et al (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2(2):141–150
Rustad KC, Gurtner GC et al (2012) Mesenchymal stem cells home to sites of injury and inflammation. Advances in Wound Care 1(4): 147–52. /pmc/articles/PMC3623614/?report=abstract (October 11, 2020)
Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N et al (2019) Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther 10(1)
Regie Santos-Cortez, Lyn P, Ying Hu, Fanyue Sun, Fairouz Benahmed-Miniuk, Jian Tao, Kanaujiya Jitendra K, Samuel Ademola, Solomon Fadiora, Victoria Odesina, Nickerson Deborah A, Bamshad Michael J, Olaitan Peter B, Oluwatosin Odunayo M, Leal Suzanne M, Reichenberger Ernst J et al (2017) Identification of ASAH1 as a susceptibility gene for familial keloids. Eur J Hum Gen 25(10):1155–61
Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H et al (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 180(4): 2581–87. https://pubmed.ncbi.nlm.nih.gov/18250469/ (October 11, 2020)
Shepherd AJ, Cloud ME, Cao YQ, Mohapatra DP et al (2018) Deficits in burrowing behaviors are associated with mouse models of neuropathic but not inflammatory pain or migraine. Front Behav Neurosci 12
Song C, Wu H-G, Chang H, Kim IH, Ha SW et al (2014) Adjuvant single-fraction radiotherapy is safe and effective for intractable keloids. J rad res 55(5):912–916
Sood RF, Hocking AM, Muffley LA, Ga M, Honari S, Reiner AP, Gibran NS et al (2015) Genome-wide association study of postburn scarring identifies a novel protective variant. Ann Surg 262(4):563–569
Spiekman M, Przybyt E, Plantinga JA, Gibbs S, van Der Lei B, Harmsen MC et al (2014) Adipose tissue-derived stromal cells inhibit TGF-Β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg 134(4):699–712
Steinstraesser L, Flak E, Witte B, Ring A, Tilkorn D, Hauser J, Langer S, Steinau HU, Al-Benna S et al (2011) Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg 128(4):306e–313e
Suzuki K, Chosa N, Sawada S, Takizawa N, Yaegashi T, Ishisaki A et al (2017) Enhancement of anti-inflammatory and osteogenic abilities of mesenchymal stem cells via cell-to-cell adhesion to periodontal ligament-derived fibroblasts. Stem cells int 2017:3296498
Tappenbeck N, Schröder HM, Niebergall-Roth E, Hassinger FD, Ulf D, Kathrin K, Korinna K, Andreas E, Jasmina F, Natasha Y, Scharffetter-Kochanek, Karin M, George F, Orgill DP, Beck J, Frank MH, Ganss C, Kluth MA et al (2019) In vivo safety profile and biodistribution of gmp-manufactured human skin-derived ABCB5-Positive Mesenchymal Stromal Cells for Use in Clinical Trials. Cytotherapy 21(5): 546–60. /pmc/articles/PMC6513723/?report=abstract (October 11, 2020)
Der Veer V, Willem M, Niessen FB, Ferreira JA, Zwiers PJ, Jong De, Etty H, Middelkoop E, Molema G et al (2011) Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen 19(3):292–301
Wilson AM (2013) Eradication of keloids: surgical excision followed by a single injection of intralesional 5-fluorouracil and botulinum toxin. Can J Plast Surg 21(2):87–91
Wu M, Ji S, Xiao S, Kong Z, Fang H, Zhang Y, Ji K, Zheng Y, Liu H, Xia Z et al (2015) JAM-A promotes wound healing by enhancing both homing and secretory activities of mesenchymal stem cells. Clin Sci 129(7): 575–88. https://pubmed.ncbi.nlm.nih.gov/25994236/ (October 11, 2020)
Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, Wells A et al (2010) Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 176(4):1743–1755
Yates CC, Nuschke A, Rodrigues M, Whaley D, Dechant JJ, Taylor DP, Wells A et al (2017) Improved transplanted stem cell survival in a polymer gel supplemented with tenascin c accelerates healing and reduces scarring of murine skin wounds. Cell Transplant 26(1):103–113
Yates CC, Rodrigues M, Nuschke A, Johnson ZI, Whaley D, Stolz D, Newsome J, Wells A et al (2017) Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther 8(1)
Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W, Rangel EB, McNiece I, Rundek T, Sacco RL, Perez-Pinzon M, Hare JM et al (2014) Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 9(5)
Yoshida Ken, Nakashima Ayumu, Doi Shigehiro, Ueno Toshinori, Okubo Tomoe, Kawano Ki, ichiro Kanawa Masami, Yukio Kato, Yukihito Higashi, Takao Masaki et al (2018) Serum-Free Medium Enhances the Immunosuppressive and Antifibrotic Abilities of Mesenchymal Stem Cells Utilized in Experimental Renal Fibrosis. Stem Cells Transl Med 7(12):893–905
Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG et al (2015) Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther 6(1)
Zhu F, Wu B, Li P, Wang J, Tang H, Liu Ye, Zuo X, Cheng H, Ding Y, Wang W, Zhai Y, Qian F, Wang W, Yuan X, Wang J, Ha W, Hou J, Zhou F, Wang Y et al (2013) Association study confirmed susceptibility loci with keloid in the Chinese Han population. PLoS ONE 8(5):e62377
Zhu KQ, Engrav LH, Tamura RN, Cole JA, Muangman P, Carrougher GJ, Gibran NS et al (2004) Further similarities between cutaneous scarring in the female, red duroc pig and human hypertrophic scarring. Burns 30(6):518–530
Zhu KQ, Engrav LH, Gibran NS, Cole JK, Matsumura H, Piepkorn M, Isik FF, Carrougher GJ, Muangman PM, Yunusov MY, Yang T-M et al (2003) The female, red duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns 29(7):649–664
Author information
Authors and Affiliations
Contributions
CB and KT contributed equally as first author. The study was designed and supervised by KT, WK, and CMM. JS and AH carried out study quality assessment. KTS conducted qualitative data synthesis. All authors contributed significantly and were involved in editing, reviewing, and approving the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bojanic, C., To, K., Hatoum, A. et al. Mesenchymal stem cell therapy in hypertrophic and keloid scars. Cell Tissue Res 383, 915–930 (2021). https://doi.org/10.1007/s00441-020-03361-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-020-03361-z