Abstract
CuCeTiOx (CCT) catalyst is considered as a promising prospect attributable to their high activity for low-temperature CO oxidation. However, rapid deactivation when treating humid flue gas hindered their industrial exploitation. The hydroxide ion (OH−) dissociated from H2O, and carbonate intermediates derived from CO/CO2 deposited on the catalyst surface of CCT catalyst, inhibits the CO oxidation by surface oxygen on active sites. In this study, the detrimental effect caused by H2O and CO2 were evaluated, and the performance of CCT catalysts were investigated and compared using in situ DRIFTs study. Further, intentional doping on the CCT using transition metal (e.g., Co and Mn) was performed to mitigate the catalyst deactivation caused by H2O and CO2. The incorporation of cobalt in Co-CCT altered the reaction pathway and mitigated the deactivation via enhancing the consumption of surface adsorbed OH- by CO, reducing the occupancy of active sites. Also, preferential adsorption of CO further suppressed the competition of OH- and CO2 towards active sites on catalyst attributable to the abundant oxygen vacancies and low coordinated metal (i.e., Cu+, Ce3+) in Co-CCT, which significantly enhanced the resistance to H2O and CO2 in the flue gas. This work thoroughly analyzed the mechanism of H2O and CO2 impacting the catalyst activity during low-temperature CO oxidation, is able to provide innovative insights for the design of highly-active and long-shelf life catalysts.
Graphic Abstract
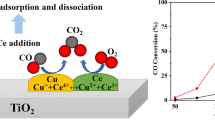
The incorporation of cobalt in CuCeTiOx catalyst facilitates the formation of oxygen vacancies, the adsorption of CO, and the consumption of OH-, speeding up the CO oxidation to CO2 and promoting the resistance to deactivation caused by H2O and CO2 in the flue gas.







Similar content being viewed by others
References
Arachchige USPR, Melaaen MC (2012) Aspen plus simulation of CO2 removal from coal and gas fired power plants. Energy Procedia 23:391–399
Huang TJ, Tsai DH (2003) CO oxidation behavior of copper and copper oxides. Catal Lett 87:173–178
Eren B, Heine C, Bluhm H et al (2015) Catalyst Chemical State during CO Oxidation Reaction on Cu(111) Studied with Ambient-Pressure X-ray Photoelectron Spectroscopy and Near Edge X-ray Adsorption Fine Structure Spectroscopy. J Am Chem Soc 137:11186–11190
Lee HC, Kim DH (2008) Kinetics of CO and H2 oxidation over CuO-CeO2 catalyst in H2 mixtures with CO2 and H2O. Catal Today 132:109–116
Chen CS, You JH, Lin JH, Chen YY (2008) Effect of highly dispersed active sites of Cu/TiO2 catalyst on CO oxidation. Catal Commun 9:2381–2385
Bagheri S, Muhd Julkapli N, Bee Abd Hamid S (2014) Titanium Dioxide as a Catalyst Support in Heterogeneous Catalysis. Sci World J 2014:1–21
Chen CSHCC, Chen TC, Chen CSHCC et al (2012) Effect of Ti3+ on TiO2-supported Cu catalysts used for CO oxidation. Langmuir 28:9996–10006
Huang J, Wang S, Zhao Y et al (2006) Synthesis and characterization of CuO/TiO2 catalysts for low-temperature CO oxidation. Catal Commun 7:1029–1034
Wu G, Guan N, Li L (2011) Low temperature CO oxidation on Cu-Cu2O/TiO2 catalyst prepared by photodeposition. Catal Sci Technol 1:601–608
Yang BL, Chan SF, Chang WS, Chen YZ (1991) Surface enrichment in mixed oxides of Cu Co, and Mn, and its effect on CO oxidation. J Catal 130:52–61
Bae J, Shin D, Jeong H et al (2019) Highly water-resistant La-doped Co3O4 catalyst for co oxidation. ACS Catal 9:10093–10100
Li C, Yang Y, Ren W et al (2020) Effect of Ce Doping on Catalytic Performance of Cu/TiO2 for CO Oxidation. Catal Letters 150:2045–2055
Chen CS, Chen TC, Wu HC et al (2020) The influence of ceria on Cu/TiO2 catalysts to produce abundant oxygen vacancies and induce highly efficient CO oxidation. Catal Sci Technol 10:4271–4281
Xie X, Li Y, Liu ZQ et al (2009) Low-temperature oxidation of CO catalysed by Co3O4 nanorods. Nature 458:746–749
Jampaiah D, Venkataswamy P, Coyle VE et al (2016) Low-temperature CO oxidation over manganese, cobalt, and nickel doped CeO2 nanorods. RSC Adv 6:80541–80548
Hoflund GB, Gardner SD, Schryer DR et al (1995) Effect of CO2 on the Performance of Au/MnOx and Pt/SnOx Low-Temperature CO Oxidation Catalysts. Langmuir 11:3431–3434
Chen A, Yu X, Zhou Y et al (2019) Structure of the catalytically active copper–ceria interfacial perimeter. Nat Catal 2:334–341
Wang Q, Li Z, Bañares MA et al (2019) A Novel Approach to High-Performance Aliovalent-Substituted Catalysts—2D Bimetallic MOF-Derived CeCuOx Microsheets. Small 15:1903525
Apopei P, Catrinescu C, Teodosiu C, Royer S (2014) Mixed-phase TiO2 photocatalysts: Crystalline phase isolation and reconstruction, characterization and photocatalytic activity in the oxidation of 4-chlorophenol from aqueous effluents. Appl Catal B Environ 160–161:374–382
Yang J, Hu S, Fang Y et al (2019) Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature co oxidation. ACS Catal 9:9751–9763
Cychosz KA, Thommes M (2018) Progress in the Physisorption Characterization of Nanoporous Gas Storage Materials. Engineering 4:559–566
Zhu C, Osherov A, Panzer MJ (2013) Surface chemistry of electrodeposited Cu2O films studied by XPS. Electrochim Acta 111:771–778
Lykaki M, Pachatouridou E, Carabineiro SAC et al (2018) Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl Catal B Environ 230:18–28
Xiong Y, Chen S, Ye F et al (2015) Synthesis of a mixed valence state Ce-MOF as an oxidase mimetic for the colorimetric detection of biothiols. Chem Commun 51:4635–4638
Yu J, Yu J, Wei Z et al (2019) Preparation and Characterization of UiO-66-Supported Cu–Ce Bimetal Catalysts for Low-Temperature CO Oxidation. Catal Lett 149:496–506
El Kabouss K, Kacimi M, Ziyad M et al (2006) Cobalt speciation in cobalt oxide-apatite materials: structure–properties relationship in catalytic oxidative dehydrogenation of ethane and butan-2-ol conversion. J Mater Chem 16:2453–2463
Petitto SC, Marsh EM, Carson GA, Langell MA (2008) Cobalt oxide surface chemistry: The interaction of CoO(100), Co3O4(110) and Co3O4(111) with oxygen and water. J Mol Catal A Chem 281:49–58
Zhao F, Li S, Wu X et al (2019) Synergetic effect over flame-made manganese doped CuO–CeO2 nanocatalyst for enhanced CO oxidation performance. RSC Adv 9:2343–2352
Nesbitt HW, Banerjee D (1998) Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2 precipitation. Am Mineral 83:305–315
Chen K, Li W, Zhou Z et al (2020) Hydroxyl groups attached to Co2+ on the surface of Co3O4: A promising structure for propane catalytic oxidation. Catal Sci Technol 10:2573–2582
Benkoula S, Sublemontier O, Patanen M et al (2015) Water adsorption on TiO2 surfaces probed by soft X-ray spectroscopies: bulk materials vs. isolated nanoparticles. Sci Rep 5:15088
Mock SA, Sharp SE, Stoner TR et al (2016) CeO2 nanorods-supported transition metal catalysts for CO oxidation. J Colloid Interface Sci 466:261–267
Mo S, Li S, Xiao H et al (2018) Low-temperature CO oxidation over integrated penthorum chinense-like MnCo2O4 arrays anchored on three-dimensional Ni foam with enhanced moisture resistance. Catal Sci Technol 8:1663–1676
Vovchok D, Guild CJ, Llorca J et al (2017) Cu supported on mesoporous ceria: water gas shift activity at low Cu loadings through metal-support interactions. Phys Chem Chem Phys 19:17708–17717
Davó-Quiñonero A, Navlani-García M, Lozano-Castelló D et al (2016) Role of hydroxyl groups in the preferential oxidation of CO over copper oxide-cerium oxide catalysts. ACS Catal 6:1723–1731
Venkov T, Hadjiivanov K (2003) FTIR study of CO interaction with Cu/TiO2. Catal Commun 4:209–213
Mo S, Zhang Q, Sun Y et al (2019) Gaseous CO and toluene co-oxidation over monolithic core-shell Co3O4-based hetero-structured catalysts. J Mater Chem A 7:16197–16210
Korhonen ST, Calatayud M, Krause Outi I, A, (2008) Structure and stability of formates and carbonates on monoclinic zirconia: a combined study by density functional theory and infrared spectroscopy. J Phys Chem C 112:16096–16102
Chen S, Cao T, Gao Y et al (2016) Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J Phys Chem C 120:21472–21485
Ma C, Yang C, Wang B et al (2019) Effects of H2O on HCHO and CO oxidation at room-temperature catalyzed by MCo2O4 (M=Mn, Ce and Cu) materials. Appl Catal B Environ 254:76–85
Zhao Y, Teng BT, Wen XD et al (2012) Superoxide and peroxide species on CeO2(111), and their oxidation roles. J Phys Chem C 116:15986–15991
Zhang Z, Wang S-S, Song R et al (2017) The most active Cu facet for low-temperature water gas shift reaction. Nat Commun 8:488
Yan Z, Yang H, Ouyang J, Tang A (2017) In situ loading of highly-dispersed CuO nanoparticles on hydroxyl-group-rich SiO2-AlOOH composite nanosheets for CO catalytic oxidation. Chem Eng J 316:1035–1046
Yin C, Liu Y, Xia Q et al (2019) Oxygen vacancy-rich nitrogen-doped Co3O4 nanosheets as an efficient water-resistant catalyst for low temperature CO oxidation. J Colloid Interface Sci 553:427–435
Di Benedetto A, Landi G, Lisi L, Russo G (2013) Role of CO2 on CO preferential oxidation over CuO/CeO2 catalyst. Appl Catal B Environ 142–143:169–177
Cox DF, Schulz KH (1991) Interaction of CO with Cu+ cations: CO adsorption on Cu2O(100). Surf Sci 249:138–148
Acknowledgments
The authors thank the financial support of the National Key Research and Development Program of China (2017YFC0210302).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, K., Wang, Q. & Wei, J. A Robust Cu Catalyst for Low-Temperature CO Oxidation in Flue Gas: Mitigating Deactivation via Co-Doping. Catal Lett 151, 2302–2312 (2021). https://doi.org/10.1007/s10562-020-03471-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-020-03471-x