Abstract
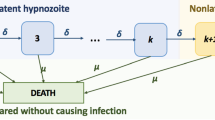
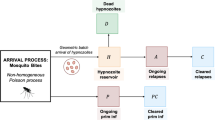
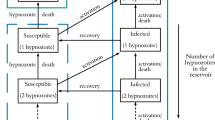
Malaria is a mosquito-borne disease that, despite intensive control and mitigation initiatives, continues to pose an enormous public health burden. Plasmodium vivax is one of the principal causes of malaria in humans. Antibodies, which play a fundamental role in the host response to P. vivax, are acquired through exposure to the parasite. Here, we introduce a stochastic, within-host model of antibody responses to P. vivax for an individual in a general transmission setting. We begin by developing an epidemiological framework accounting for P. vivax infections resulting from new mosquito bites (primary infections), as well as the activation of dormant-liver stages known as hypnozoites (relapses). By constructing an infinite server queue, we obtain analytic results for the distribution of relapses in a general transmission setting. We then consider a simple model of antibody kinetics, whereby antibodies are boosted with each infection, but are subject to decay over time. By embedding this model for antibody kinetics in the epidemiological framework using a generalised shot noise process, we derive analytic expressions governing the distribution of antibody levels for a single individual in a general transmission setting. Our work provides a means to explore exposure-dependent antibody dynamics for P. vivax, with the potential to address key questions in the context of serological surveillance and acquired immunity.







Similar content being viewed by others
References
Ademolue TW, Awandare GA (2018) Evaluating antidisease immunity to malaria and implications for vaccine design. Immunology 153(4):423–434
André D, Silva Júlio CAL, Antony S, Paola Marchesini CJ, Ter Kuile FFO, Lalloo DG (2019) Evaluation of Plasmodium vivax malaria recurrence in Brazil. Malaria J 18(1):18
Battle KE, Karhunen MS et al (2014) Geographical variation in Plasmodium vivax relapse. Malaria J 13(1):1–16
Battle KE, Lucas TCD et al (2019) Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet 394(10195):332–343
Beier JC, Beier MS, Vaughan JA, Pumpuni CB, Davis JR, Noden BH (1992) Sporozite transmission by anopheles freeborni and anopheles gambiae experimentally infected with plasmodium falciparum. J Am Mosquito Control Assoc 8:404–404
Chatterjee U, Mukherjee SP (1989) On the non-homogeneous service system MX/G/$\infty $. Eur J Oper Res 38(2):202–207
Drakeley CJ et al (2005) Estimating medium-and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Nat Acad Sci 102(14):5108–5113
Fowkes FJI, Boeuf P, Beeson JG (2016) Immunity to malaria in an era of declining malaria transmission. Parasitology 143(2):139–153
Franca CT et al (2016) An antibody screen of a Plasmodium vivax antigen library identifies novel merozoite proteins associated with clinical protection. PLoS Neglect Trop Dis 10(5):e0004639
Gomes PS, Bhardwaj J, Rivera-Correa J, Freire-De-Lima CG, Morrot A (2016) Immune escape strategies of malaria parasites. Front Microbiol 7:1617
Greenhouse B et al (2019) Priority use cases for antibody-detecting assays of recent malaria exposure as tools to achieve and sustain malaria elimination. In: Gates Open Research 3
He WQ, Karl S et al (2019) Antibodies to Plasmodium vivax reticulocyte binding protein 2b are associated with protection against P. vivax malaria in populations living in low malaria transmission regions of Brazil and Thailand. PLoS Neglect Trop Dis 13(8):e0007596
He W-Q, Shakri AR et al (2019) Antibody responses to Plasmodium vivax Duffy binding and Erythrocyte binding proteins predict risk of infection and are associated with protection from clinical malaria. PLoS Neglect Trop Dis 13(2):e0006987
Holman DF, Chaudhry ML, Kashyap BRK (1983) On the service system MX/G/$\infty $. Eur J Oper Res 13(2):142–145
Lewis PAW (1967) Non-homogeneous branching Poisson processes. J R Stat Soc Ser B (Methodological) 29(2):343–354
Lewis PAW, Shedler GS (1979) Simulation of nonhomogeneous Poisson processes by thinning. Naval Res Logist Quart 26(3):403–413
Longley RJ, França CT et al (2017) Asymptomatic Plasmodium vivax infections induce robust IgG responses to multiple blood-stage proteins in a low-transmission region of western Thailand. Malaria J 16(1):178
Longley RJ, White MT et al (2017) Naturally acquired antibody responses to more than 300 Plasmodium vivax proteins in three geographic regions. PLoS Neglect Trop Dis 11(9):e0005888
López C, Yepes-Pérez Y, Hincapié-Escobar N, Dıaz-Arévalo D, Patarroyo MA (2017) What is known about the immune response induced by Plasmodium vivax malaria vaccine candidates? Front Immunol 8:126
Mehra S, McCaw JM, Flegg MB, Taylor PG, Flegg JA (2020) An activation-clearance model for Plasmodium vivax malaria. Bull Math Biol 82(2):32
Mikolajczak SA et al (2015) Plasmodium vivax liver stage development and hypnozoite persistence in human liver-chimeric mice. Cell Host Microbe 17(4):526–535
Mueller I, Galinski MR, Kevin Baird J, Carlton JM, Kochar DK, Alonso PL, del Portillo HA (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9(9):555–566
Mueller IVO, Mary RG Takafumi T, Myriam A-H, William EC, Christopher LK (2013) Natural acquisition of immunity to Plasmodium vivax: epidemiological observations and potential targets. In: Advances in parasitology, vol 81. Elsevier, pp 77–131
Naing C, Maxine AW, Victor NW, Joon WM (2014) Is Plasmodium vivax malaria a severe malaria? A systematic review and meta-analysis. PLoS Neglect Trop Dis 8(8):e3071
Parzen E (1999) Stochastic processes, vol 24. In: SIAM, Philadelphia
Pires CV et al (2018) Blood-stage Plasmodium vivax antibody dynamics in a low transmission setting: a nine year follow-up study in the Amazon region. PLoS ONE 13(11):e0207244
Pothin E, Ferguson NM, Drakeley CJ, Ghani AC (2016) Estimating malaria transmission intensity from Plasmodium falciparum serological data using antibody density models. Malaria J 15(1):79
Schofield L, Mueller I (2006) Clinical immunity to malaria. Curr Mol Med 6(2):205–221
Struik SS, Riley EM (2004) Does malaria suffer from lack of memory? Immunol Rev 201(1):268–290
Taylor RR, Egan A, McGuinness D, Jepson A, Adair R, Drakely C, Riley E (1996) Selective recognition of malaria antigens by human serum antibodies is not genetically determined but demonstrates some features of clonal imprinting. Int Immunol 8(6):905–915
Tham W-H, Beeson JG, Rayner JC (2017) Plasmodium vivax vaccine research-we’ve only just begun. Int J Parasitol 47(2–3):111–118
Wardrop NA, Barnett AG, Atkinson J-A, Clements ACA (2013) Plasmodium vivax malaria incidence over time and its association with temperature and rainfall in four counties of Yunnan Province, China. Malaria J 12(1):452
Weber GE et al (2017) Sero-catalytic and antibody acquisition models to estimate differing malaria transmission intensities in Western Kenya. Sci Rep 7(1):16821
White NJ, Mallika I (2012) Relapse. In: Advances in parasitology, vol 80. Elsevier, pp 113–150
White MT, Griffin JT, Akpogheneta O, Conway DJ, Koram KA, Riley EM, Ghani AC (2014) Dynamics of the antibody response to Plasmodium falciparum infection in African children. J Infect Dis 210(7):1115–1122
White MT, Stephan K, Katherine EB, Simon IH, Ivo M, Azra CG (2014) Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. eLife 3:e04692
Yman V, White MT, Rono J et al (2016) Antibody acquisition models: a new tool for serological surveillance of malaria transmission intensity. Sci Rep 6:19472
Yman V, White MT, Asghar M, Sundling C, Sondén K, Draper SJ, Osier FHA, Färnert A (2019) Antibody responses to merozoite antigens after natural Plasmodium falciparum infection: kinetics and longevity in absence of re-exposure. BMC Med 17(1):22
Acknowledgements
S. Mehra acknowledges funding from the Australian Mathematical Sciences Institute (AMSI) Vacation Research Scholarships 2018/2019. J.M. McCaw’s research is supported by the Australian Research Council (ARC) Discovery Project DP170103076. J.A. Flegg’s research is supported by the ARC DECRA Fellowship DE160100227. P.G. Taylor’s research is supported by the ARC Laureate Fellowship FL130100039 and the ARC Centre of Excellence for the Mathematical and Statistical Frontiers (ACEMS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Recall that \(\varvec{\omega }(t)\) denotes the antibody response to a panel of proteins \(V_1, \dots , V_R\) time t after a single infection, with
from Eq. (9), with \(b_i, \eta _i > 0\) for each i.
Given that the distribution of \(\eta _i\) decays exponentially for each i, the MGF of \(\varvec{\omega }(t)\) is well defined in an open hypersphere around the origin. We seek to show that the inequality
also holds for an open hypersphere around \(\varvec{s}=\varvec{0}\).
By noting that \(G(u)=\mathrm{d}B(u)/\mathrm{d}u\) (Eq. 13), condition (39) can equivalently be written
Since \(\varvec{\omega }(t) \le \varvec{\omega }(0)\) for all \(t \ge 0\),
noting that the probability mass \(B(t) \in [0, 1]\) for all \(t \ge 0\).
By assumption, \({\mathbb {E}} \big [e^{ \varvec{\omega }(0) \cdot \varvec{s}} \big ]\), the MGF of \(\varvec{\omega }(0) = (\eta _1, \dots , \eta _R)\) is well defined in some open hypersphere around the origin. It follows that \({\mathbb {E}} \big [e^{ \varvec{\omega }(0) \cdot \varvec{s}} \big ]\) is differentiable, therefore continuous at the origin with respect to \(\varvec{s}\), hence, there exists \(\delta >0\) such that
that is, the condition specified in Eq. (39) is satisfied in an open hypersphere around the origin, as required.
Rights and permissions
About this article
Cite this article
Mehra, S., McCaw, J.M., Flegg, M.B. et al. Antibody Dynamics for Plasmodium vivax Malaria: A Mathematical Model. Bull Math Biol 83, 6 (2021). https://doi.org/10.1007/s11538-020-00837-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00837-5