Abstract
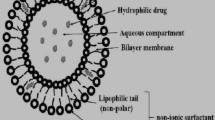
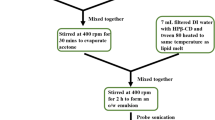
Curcumin is a natural polyphenol obtained from Curcuma longa which has gained much attention in medical and scientific fields and is known for its wide range of therapeutic applications. But the natural herb mixtures with high subatomic sizes have low retention limits which does not permit the restorative medication to cross the lipid membranes, subsequently bringing about low bioavailability with confined proficiency. Nanobiotechnology has overcome the drawbacks of curcumin delivery to its targeted site through the use of antioxidant-rich, biodegradable SLNs as drug delivery vehicle with specificity and expanded bioavailability that will encourage the dynamic release of therapeutic ingredients into the body. Herein, optimized curcumin-loaded SLNs (CuSLNs) were developed. The morphological analysis of SEM has shown the average particle size was found to be in the range of 71–112 nm. The physicochemical characteristics of optimized CuSLNs were particle size of 106 nm, zeta potential of − 54 mV, polydispersity index of 0.429 which have confirmed the nanoparticle stability. The FTIR, DSC, and XRD results have suggested the lipids are compatible and confirmed the complete solubilization of curcumin in the lipid phase. Encapsulation efficiency and drug loading capacity of optimized formulation were 88.22 and 13.13, respectively. In vitro release studies have shown the controlled drug release rate of 88% compared with free curcumin and 81% of in vitro antioxidant activity which is compared with the ascorbic acid. The studies confirmed that the formulation of CuSLNs is a promising drug delivery system with controlled drug release, improved bioavailability with good stability.












Similar content being viewed by others
References
Augustine A, Pius A, Gopi S, Gopi S (2017) Biological activities of Curcuminoids, other bio molecules from turmeric and their derivatives-a review. J Tradit Complement Med 7:205–233
Anand P, Thomas SG, Ajaikumar B, Kunnumakkara AB, Sundaram C, Harikumar KB (2008) Biological activities of Curcumin and its analogues (Congeners) made by man and mother nature. BiochemPharmacol 7:1590–1611
Rahman I, Adcock IM (2006) Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 28(219):42
Fiala M, Liu PT, EspinosaJeffrey A, Rosenthal MJ, Bernard G, Ringman JM (2007) Innate immunity and transcription of MGAT-III and Tolllike receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci USA 104:12849–12854
Anand P et al (2007) Bioavailability of Curcumin: problems and promises. Mol Pharm 4:807–818
Jager R et al (2014) Comparative absorption of curcumin formulations. Nutr J 13:11
Mignani S, El Kazzouli S, Bousmina M, Majoral JP (2013) Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev 65:1316–1330
Biswas CS, Biswas A, Galluzzi M, Shekh MI, Wang Q, Ray B, Maiti P, Stadler FJ (2020) Synthesis and characterization of novel amphiphilic biocompatible block-copolymers of poly (N-isopropylacrylamide)-b-poly (l-phenylalanine methyl ester) by RAFT polymerization. Polymer 203:122760
Shekh MI, Amirian J, Stadler FJ, Du B, Zhu Y (2020) Oxidized chitosan modified electrospun scaffolds for controllable release of acyclovir. Int J Biol Macromol 151:787–796
Dhanavel S, Revathy TA, Sivaranjani T, Sivakumar K, Palani P, Narayanan V, Stephen A (2020) 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines. Polym Bull 77(1):213–233
Dhanavel S, Praveena P, Narayanan V, Stephen A (2019) Chitosan/reduced graphene oxide/Pd nanocomposites for co-delivery of 5-fluorouracil and Curcumin towards HT-29 colon cancer cells. Polym Bull 5:1–6
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S (2015) Advances and challenges of liposome assisted drug delivery. Front Pharm 6:286
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K (2013) Liposome: classification, preparation, and applications. Nanoscale Res Lett 8:102
Zylberberg C, Matosevic S (2016) Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv 23:3319–3329
Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, Stewart MH, Medintz IL (2013) Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev 113:1904–2074
Zhang L, Gu F, Chan J, Wang A, Langer R, Farokhzad O (2008) Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther 83:761–769
Miyata K, Christie RJ, Kataoka K (2011) Polymeric micelles for nano-scale drug delivery. React Funct Polym 71:227–234
Xu W, Ling P, Zhang T (2013) Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv 2013:340315
Kesharwani P, Xie L, Banerjee S, Mao G, Padhye S, Sarkar FH, Iyer AK (2015) Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3, 4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Coll Surf B 136:413–423
Zhu J, Shi X (2013) Dendrimer-based nanodevices for targeted drug delivery applications. J Mater Chem B 1:4199–4211
Madaan K, Kumar S, Poonia N, Lather V, Pandita D (2014) Dendrimers in drug delivery and targeting: drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci 6:139
Kesharwani P, Jain K, Jain NK (2014) Dendrimer as nanocarrier for drug delivery. Progr Polym Sci 39:268–307
Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A, Larrea G, Sosa- Ferreyra C, Mercado-Curiel R, Manríquez J, Bustos E (2014) Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomater 2014:39
McNamara K, Tofail SA (2015) Nanosystems: the use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys Chem Chem Phys 17:27981–27995
Kudr J, Haddad Y, Richtera L, Heger Z, Cernak M, Adam V, Zitka O (2017) Magnetic nanoparticles: from design and synthesis to real world applications. Nanomaterials 7:243
Zebib B, Mouloungui Z, Noirot V (2010) Stabilization of Curcumin by complexation with divalent cations in glycerol/water system. Bioinorg Chem Appl. https://doi.org/10.1155/2010/292760
Zhao ZX, Jiang T, Wang L, Yang H, Zhang S, Zhou P (2010) Interaction of Curcumin with Zn (II) and Cu (II) ions based on experiment and theoretical calculation. J Mol Struct 984:316–325
Guo M, Muhammad F, Wang A, Qi W, Wang N, Guo Y, Weic Y, Zhu G (2013) Magnesium hydroxide nanoplates: a pH-responsive platform for hydrophobic anticancer drug delivery. J Mater Chem B 1:5273–5278
Adib M, Ghanbarzadeh S, Kouhsoltani M, Khosroshahi AY, Hamishehkar H (2016) The effect of particle size on the deposition of solid lipid nanoparticles in different skin layers: a histological study. Adv Pharm Bull 6:31–36
Manjunath K, Reddy JS, Venkateswarlu V (2005) Solid lipid nanoparticles as drug delivery systems. Methods Find Exp Clin Pharmacol 27:127–144
Ekambaram P, Sathali A, Priyanka K (2012) Solid lipid nanoparticles: a review. Sci Rev Chem Commun 2:80–102
Ramteke KH, Joshi SA, Dhole SN (2012) Solid lipid nanoparticle: a review. IOSR J Pharm 2:34–44
Müller RH, Shegokar R, Keck CM (2011) 20 years of lipid nanoparticles (SLN and NLC) present state of development and industrial applications. Curr Drug Discov Technol 8:207–227
Mühlen A, Schwarz C, Mehnert W (1998) Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm 45:149–155
Chen J, Stavro PM, Thompson LU (2002) Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer 43:187–192
Wiesenborn D, Kangas N, Tostenson K, Hall KCIII, Chang K (2005) Sensory and oxidativequality of screw-pressed flaxseed oil. J Am Oil Chem Soc 82:887–892
Wang L, Chen J, Thompson LU (2005) The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenografts is attributed to both its lignan and oil components. Int J Cancer 116:793–798
Dwivedi C, Natarajan K, Matthees DP (2005) Chemopreventive effects of dietary flaxseedoil on colon tumour development. Nutr Cancer 51:52–58
Prasad K (1997) Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 132:69–76
Xu J, Yang W, Deng Q, Huang Q, Yang J, Huang F (2012) Flaxseed oil and a-lipoic acid combination reduces atherosclerosis risk factors in rats fed a high-fat diet. Lipids Health Dis 11:148
Üner M, Yener G (2007) Importance of solid lipid nanoparticles (SLN) in various administration routes and future perspectives. Int J Nanomed 2:289–300
Triplett DM, Rathman FJ (2007) Optimization of β- carotene loaded solid lipid nanoparticles preparation using a high shear homogenization technique. J Nanoparticle Res 11:601–614
Yassin AE, Anwer MK, Mowafy HA, El-Bagory IM, Bayomi MA, Alsarra IA (2010) Optimization of 5-flurouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer. Int J Med Sci 7:398–408
Rahman SMH, Telny TC, Ravi TK, Kuppusamy S (2009) Role of surfactant and pH in dissolution of Curcumin. Ind J Pharm Sci 71:139–142
Cheel J, Theoduloz C, Rodriguez J, Schmeda- Hirschmann G (2005) Free radical scavengersand antioxidants from lemongrass (Cymbopogoncitratus (DC) Stapf). J Agric Food Chem 53:2511–2517
Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44:307–315
Chung YC, Chang CT, Chao WW, Lin CF, Chou ST (2002) Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem 50:2454–2458
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of“antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Saavedra G, Murcia MA (2003) Investigation of Bolivian plant extracts for their radical scavenging activity and antioxidant activity. Life Sci 73:1667–1681
Maksimović Z, Malenčić D, Kovačević N (2005) Polyphenol contents and antioxidant activity of maydis stigma extracts. Bio-resour Technol 96:873–877
Kumar S, Randhawa JK (2014) Paliperidone-loaded spherical solid lipid nanoparticles. RSC Adv 4:30186–30192
Lacatusu I (2013) Lipid nanoparticles based on omega-3 fatty acids as effectivecarriers for lutein delivery. Preparation and in vitro characterization studies. J Funct Foods 5:1260–1269
Michele T, Francesca D, Otto C (2003) Preparation of solid lipid nanoparticles by a solvent emulsification–diffusion technique. Int J Pharm 257:153–160
Kumar PP, Gayatri P, Sunil R, Jaganmohan S, Rao YM (2013) Atorvastatin loaded solid lipid nanoparticles: formulation, optimization, and in vitro characterization. IOSR J Pharm 2:23–32
Chavda H, Patel J, Chavada G, Dave S, Patel A, Patel C (2013) Self nanoemulsifying powder of isotretinoin: preparation and characterization. J Powder Technol 2013:108569
Rehman M, Madni A, Ihsan A, Khan WS, Khan MI, Mahmood MA, Ashfaq M, Bajwa SZ, Shakir I (2015) Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomed 10:2805
Olbrich C, Kayser O, Müller RH (2002) Lipase degradation of Dynasan 114 and 116 solid lipid nanoparticles (SLN)–effect of surfactants, storage time and crystallinity. Int J Pharm 237:119–128
Anton N, Benoit JP, Saulnier P (2008) Design and production of nanoparticles formulated fromnano-emulsion templates-a review. J Control Release 128:185–199
Zhang J, Fan JY, Smith YE (2009) Experimental design for the optimization of lipidnanoparticles. J Pharm Sci 98:1813–1819
Cavalli R, Caputo O, Gasco MR (2000) Preparation and characterization of solid lipidnanospheres containing paclitaxel. Eur J Pharm Sci 10:305–309
Heurtault B, Saulnier P, Pech H (2003) Physico-chemical stability of colloidal lipid particles. Biomaterials 24:4283–4300
Rahman MH, Ramanathan M, Sankar V (2014) Preparation, characterization and in vitro cytotoxicity assay of Curcumin loaded solid lipid nanoparticle in IMR32 neuroblastoma cell line. Pak J Pharm Sci 27:1281–1285
Clogston JD, Patri AK (2011) Zeta potential measurementMethods. Mol Biol 697:63–70
Chen J (2013) Fabrication and evaluation of Curcumin-loaded nanoparticles based on solid lipid as a new type of colloidal drug delivery system. Indian J Pharm Sci 75:178–184
Niveta S, Dipti KT, Seema C (2017) Formulation and evaluation of PEGylated GMS based solid lipid nanoparticles. Int J Biotech Biomed Sci 3:52–57
Kolev TM, Velcheva EA, Stambolyska BA, Speteller M (2005) DFT and experimental studies of the structure and vibrational spectra of Curcumin. Int J Quant Chem 102:1069–1079
Yallapu MM, Jaggi M, Chauhan SC (2010) β-Cyclodextrin-Curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Coll Surf B 79:113–125
Mei D, Zhang B, Liu R, Zhang H, Liu J (2011) Preparation of stearic acid/halloysite nanotube composite as form-stable PCM for thermal energy storage. Int J Energy Res 35:828–834
Fu X, Liu Z, Wu B, Wang J, Lei J (2016) Preparation and thermal properties of stearic acid/diatomite 9 composites as form-stable phase change materials for thermal energy storage via direct impregnation method. J Therm Anal Calorim 123:1173–1181
Wang Y, Xia TD, Zheng H, Feng HX (2011) Stearic acid/silica fume composite as form-stable phase change material for thermal energy storage. Energy Build 43:2365–2370
Zhang ZS, Wang LJ, Li D, Li SJ, Zkan ON (2011) Characteristics of flaxseed oil from two different flax plants. Int J Food Prop 14:1286–1296
Mulik RS, Monkkonen J, Juvonen RO, Mahadik KR, Paradkar AR (2010) Transferrin mediated solid lipid nanoparticles containing Curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int J pharm 398:190–203
Izumikawa S, Yoshioka S, Aso Y, Tkeda Y (1991) Preparation of poly(1-lactide) microspheres of different crystalline morphology and effect of crystalline morphology on drug release rate. J control release 15:133–140
Muller RH, Mader K, Gohla S (2000) Solid lipid nanoparticle (SLN) for controlled drugdelivery–a review of the state of the art. Eur J Pharm Biopharm 50:161–177
Jenning V, Gysler A, Schäfer-Korting M, Gohla SH (2000) Vitamin A loaded solid lipidnanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm 49:211–218
Kuo YC, Chen HH (2009) Entrapment and release of saquinavir using novel cationic solid lipid nanoparticles. Int J Pharm 365:206–213
Kuo YC, Chung JF (2011) Physicochemical properties of nevirapineloaded solid lipidnanoparticles and nanostructured lipid carriers. Coll Surf B Biointerfaces 83:299–306
Schwarz C, Mehnert W, Lucks JS (1994) Solid lipid nanoparticles (SLN) for controlled drug delivery. I. Production, characterization and sterilization. J Control Release 30:83–96
Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of Curcumin. Adv Exp Med Biol 595:105–125
Wright JS (2002) Predicting the antioxidant activity of Curcumin and curcuminoids. J Mol Struct 591:207–217
Priyadarsini KI (1997) Free radical reactions of Curcumin in model membranes. Free Radic Biol Med 23:838–884
Sun YM (2002) Theoretical elucidation on the antioxidant mechanism of Curcumin: a DFT study. Org Lett 4:2909–2911
Ak T, Gulcin I (2008) Antioxidant and radical scavenging properties of Curcumin. Chem Biol Interact 174:27–37
Acknowledgements
The authors are highly grateful to DST-CURIE Center, Sri Padmavati Mahila Visvavidyalayam, Tirupati, for extending the instrumentation facility to carry out this research work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ganna, S., Gutturu, R., Borelli, D.P. et al. Formulation, optimization, and in vitro characterization of omega-3-rich binary lipid carriers for curcumin delivery: in vitro evaluation of sustained release and its potential antioxidant behavior. Polym. Bull. 79, 307–330 (2022). https://doi.org/10.1007/s00289-020-03494-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03494-9