Abstract
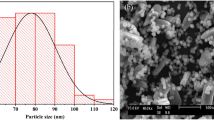
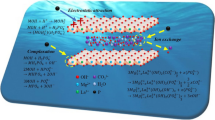
In this experiment, chitosan (CS), waste limestone and diammonium hydrogen phosphate were used as raw materials to synthesize HAp-coated-stone composites/CS by one-step blending method. Then, the prepared HAp-coated-stone composites/CS composite material was used to remove Cu (II) in the solution. The structure and microstructure of the composite were characterized by Fourier transform infrared spectroscopy, X-ray diffraction analysis, Scanning Electron Microscopy, Brunauer–Emmett–Teller and Thermogravimetric Analysis. The results showed that with the addition of CS, the overall thermal stability of the composite improved, while its specific surface area decreased. Moreover, the composite was a mesoporous material. HAp-coated-stone/CS composites with small amounts of CS (0.5%) can remarkably increase Cu (II) removal. The HAp-coated-limestone/CS-0.5% had better resistance to pH changes compared with HAp-coated-limestone. Under the initial concentration of 20 mg/L, the maximum adsorption capacity for Cu (II) adsorption by HAp-coated-limestone/CS-0.5% was about 130.75 mg/g at 40 °C. The equilibrium data and kinetic were well described by Freundlich models and pseudo-second-order, respectively. The possible mechanisms for Cu (II) adsorption onto HAp-coated-stone/CS composites surface have been proposed.










Similar content being viewed by others
References
Peng X, Chen W, He Z, Li D, Liu H, Jin H, Zhou G, Xu F (2019) Removal of Cu (II) from wastewater using doped HAP-coated-limestone. J Mol Liq 293:111502
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Pro Polym Sci 30:38–70
Fang X, Zhu S, Ma J, Wang F, Xu H, Xia M (2020) The facile synthesis of zoledronate functionalized hydroxyapatite amorphous hybrid nanobiomaterial and its excellent removal performance on Pb(II) and Cu(II). J Hazard Mater 392:122291
Al-Saydeh SA, El-Naas MH, Zaidi SJ (2017) Copper removal from industrial wastewater: A comprehensive review. J Ind Eng Chem 56:35–44
Abdel-Aziz MH, El-Ashtoukhy ESZ, Bassyouni M (2016) Recovery of copper from effluents by cementation on aluminum in a multirotating cylinder-agitated vessel. Metall Mater Trans B-Proc Metall Mater Proc Sci 47:657–665
Wang JL, Tang XB, Liang H, Bai LM, Xie BH, Xing JJ, Wang TY, Zhao J, Li GB (2020) Efficient recovery of divalent metals from nanofiltration concentrate based on a hybrid process coupling single-cation electrolysis (SCE) with ultrafiltration (UF). J Membr Sci 602:10
Zhang D, Niu Q, Ma L, Derese S, Verliefde A, Ronsse F (2020) Complete oxidation of organic waste under mild supercritical water oxidation by combining effluent recirculation and membrane filtration. Sci Total Environ 736:139731
Hube S, Eskafi M, Hrafnkelsdottir KF, Bjarnadottir B, Bjarnadottir MA, Axelsdottir S, Wu B (2020) Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci Total Environ 710:22
Rueda-Marquez JJ, Levchuk I, Ibanez PF, Sillanpaa M (2020) A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J Clean Prod 258:13
Liu Y, Chen M, Hao YM (2013) Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem Eng J 218:46–54
Ngueagni PT, Woumfo ED, Kumar PS, Siewe M, Vieillard J, Brun N, Nkuigue PF (2020) Adsorption of Cu(II) ions by modified horn core: Effect of temperature on adsorbent preparation and extended application in river water. J Mol Liq 298:112023
Arici T A, Ozcan A S,Ozcan A. Biosorption characteristics of Cu(II) and Cd(II) ions by modified alginate. J Polym Environ. Doi: https://doi.org/10.1007/s10924-020-01844-2
Tang X, Zhang Q, Liu Z, Pan K, Dong Y, Li Y (2014) Removal of Cu(II) by loofah fibers as a natural and low-cost adsorbent from aqueous solutions. J Mol Liq 199:401–407
Zeng L L, Zhou F X, Hu R, Xie W Y, Wang GX, Yang CX, Liang EX, Xu, W Y, Van der Bruggen B. Synthesis of cross-linked carboxyl modified polyvinyl alcohol and its application in selective adsorption separation of Cu(II) from Cd(II) and Ni(II). J Polym Environ DOI: 0.1007/s10924–020–01847-z
Bansode RR, Losso JN, Marshall WE, Rao RM, Portier RJ (2003) Adsorption of metal ions by pecan shell-based granular activated carbons. Bioresource Technol 89:115–119
Faisal AAH, Abdul-Kareem MB, Mohammed AK, Naushad M, Ghfar AA, Ahamad T (2020) Humic acid coated sand as a novel sorbent in permeable reactive barrier for environmental remediation of groundwater polluted with copper and cadmium ions. J Water Process Eng 36:12
Rao MM, Ramana DK, Seshaiah K, Wang MC, Chien SWC (2009) Removal of some metal ions by activated carbon prepared from phaseolus aureus hulls. J Hazard Mater 166:1006–1013
Vardikar HS, Bhanvase BA, Rathod AP, Sonawane SH (2018) Sonochemical synthesis, characterization and sorption study of Kaolin-Chitosan-TiO2 ternary nanocomposite: advantage over conventional method. Mater Chem Phys 217:457–467
Yu S, Wang X, Ai Y, Liang Y, Ji Y, Li J, Hayat T, Alsaedi A, Wang X (2016) Spectroscopic and theoretical studies on the counterion effect of Cu(II) ion and graphene oxide interaction with titanium dioxide. Environ Sci-Nano 3:1361–1368
Li J, Zhang S, Chen C, Zhao G, Yang X, Li J, Wang X (2012) Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles. ACS Appl Mater Inter 4:4991–5000
Zhao GX, Li JX, Ren XM, Chen CL, Wang XK (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Alhumaimess MS, Alsohaimi IH, Alqadami AA, Kamel MM, Naushad M, Ahamad T, Alshammari H (2019) Synthesis of phosphorylated raw sawdust for the removal of toxic metal ions from aqueous medium: Adsorption mechanism for clean approach. J Sol-Gel Sci Techno 89:602–615
Ahamad T, Naushad M, Eldesoky GE, Alqadami AA, Khan A (2019) Synthesis and characterization of egg-albumen-formaldehyde based magnetic polymeric resin (MPR): Highly efficient adsorbent for Cd(II) ion removal from aqueous medium. J Mol Liq 286:110951
Alqadami AA, Naushad M, ALOthman ZA, Alsuhybani M, Algamdi M (2020) Excellent adsorptive performance of a new nanocomposite for removal of toxic Pb(II) from aqueous environment: adsorption mechanism and modeling analysis. J Hazar Mater 389:121896
Corami A, D’Acapito F, Mignardi S, Ferrini V (2008) Removal of Cu from aqueous solutions by synthetic hydroxyapatite: EXAFS investigation. Mater Sci Eng B-Adv 149:209–213
Lin KL, Pan JY, Chen YW, Cheng RM, Xu XC (2009) Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J Hazar Mater 161:231–240
Sadat-Shojai M, Khorasani MT, Dinpanah-Khoshdargi E, Jamshidi A (2013) Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater 9:7591–7621
Suchanek W, Yoshimura M (1998) Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J Mater Res 13:94–117
Lei Y, Guan JJ, Chen W, Ke QF, Zhang CQ, Guo YP (2015) Fabrication of hydroxyapatite/chitosan porous materials for Pb(II) removal from aqueous solution. RSC Adv 5:25462–25470
Sassoni E, Naidu S, Scherer GW (2011) The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J Cult Herit 12:346–355
Long Y, Lei Y, Zhang Y, Wang S, Han Z, Shi X (2010) Determination of free calcium oxide in steel slag by EDTA complexometric titration. Metallurgical Analysis 30:65–68
Sadat-Shojai M, Atai M, Nodehi A, Khanlar LN (2010) Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent Mater 26:471–482
Wan YZ, Hong L, Jia SR, Huang Y, Zhu Y, Wang YL, Jiang HJ (2006) Synthesis and characterization of hydroxyapatite-bacterial cellulose nanocomposites. Compos Sci Technol 66:1825–1832
Li N, Bai R (2006) Highly enhanced adsorption of lead ions on chitosan granules functionalized with poly(acrylic acid). Ind Eng Chem Res 45:7897–7904
Gandhi MR, Kousalya GN, Meenakshi S (2011) Removal of copper(II) using chitin/chitosan nano-hydroxyapatite composite. Int J Biol Macromol 48:119–124
Chowdhury S, Das P (2011) Kinetic, and thermodynamic evaluation of adsorption of hazardous malachite green onto conch shell powder. Sep Sci Technol 46:1966–1976
Yang L, Zhong WH, Cui J, Wei ZG, Wei W (2016) Enhanced removal of Cu(II) ions from aqueous solution by poorly crystalline hydroxyapatite nanoparticles. J Dispersion Sci Technol 37:956–968
Rosskopfova O, Galambos M, Ometakova J, Caplovicova M, Rajec P (2012) Study of sorption processes of copper on synthetic hydroxyapatite. J Radioanal Nucl Chem 293:641–647
Vengris T, Binkien R, Sveikauskait A (2001) Nickel, copper and zinc removal from waste water by a modified clay sorbent. Appl Clay Sci 18:183–190
Sricharoen P, Limchoowong N, Areerob Y, Nuengmatcha P, Techawongstien S, Chanthai S (2017) Fe3O4/hydroxyapatite/graphene quantum dots as a novel nano-sorbent for preconcentration of copper residue in Thai food ingredients: optimization of ultrasound-assisted magnetic solid phase extraction. Ultrason Sonochem 37:83–93
Bibak A, Borggaard OK (1994) Molybdenum adsorption by aluminum and iron-oxides and humic acid. Soil Sci 25:3229–3239
Liang P, Qin YC, Hu B, Peng TY, Jiang ZC (2001) Nanometer-size titanium dioxide microcolumn on-line preconcentration of trace metals and their determination by inductively coupled plasma atomic emission spectrometry in water. Anal Chim Acta 440:207–213
Liu Y, Duan M, Yu Z (2013) Agricultural landscapes and biodiversity in China. Agr Ecosyst Environ 166:46–54
Dima JB, Sequeiros C, Zaritzky NE (2015) Hexavalent chromium removal in contaminated water using reticulated chitosan micro/nanoparticles from seafood processing wastes. Chemosphere 141:100–111
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant No. 11964018) and the Natural Science Foundation of Jiangxi Province of China (Grant No. 20181BAB202027).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Peng, X., Li, Y., Liu, S. et al. A Study of Adsorption Behaviour of Cu(II) on Hydroxyapatite-Coated-Limestone/Chitosan Composite. J Polym Environ 29, 1727–1741 (2021). https://doi.org/10.1007/s10924-020-02009-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-02009-x