Abstract
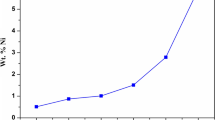
Different concentrations of Citrus aurantium with deoxyribonucleic acid as additive (Citrus aurantiumDNA) were co-deposited with nickel via electrodeposition. The coatings were formed in an aqueous nickel bath with the incorporation of Citrus aurantiumDNA concentrations at different temperatures, and the influence of the acid and the temperature on the microstructure of the coatings was studied. The data via adsorption isotherms were also analyzed. The characteristics of the morphological structure of the coatings were examined by scanning electron microscopy. The results demonstrated that Citrus aurantiumDNA additives were evenly dispersed on the mild steel surface during the nickel coatings deposition; a current density of 1.5 A/dm3 used during the electrolysis process was favourable in attaining the maximum coating efficiency (~70%) at 25°C and 20 mg/L of Citrus aurantiumDNA. The optimum temperature for electroplating was found to be 25°C. The data spawned from the adsorption Freundlich and Langmuir isotherms showed two preferred temperatures: i.e. 25 and 40°C via the former and 55 and 70°C via the latter. The thermodynamic assessment confirms that the adsorption mechanism of the Citrus aurantiumDNA additive in a Ni electrolyte solution on mild steel in an HCl solution is physisorption at the investigated temperatures. X-ray diffraction revealed some broad peaks signifying an amorphous structure at all Citrus aurantiumDNA concentrations.












Similar content being viewed by others
Change history
06 May 2021
An Erratum to this paper has been published: https://doi.org/10.3103/S1068375521020150
REFERENCES
Sadiku-Agboola, O., Sadiku, E.R., Ojo, O.I., Akanji, O.L., et al., Port. Electrochim. Acta, 2011, vol. 29, pp. 91–100.
Di Bari, G.A., Electrodeposition of nickel, in Modern Electroplating, Schlesinger, M. and Paunovic, M., Eds., Chichester: Wiley, 2010, 5th ed., p. 79. http://www.tuks.nl/WFCProject/pdf/Electrodepositon_of_Nickel.pdf.
Kilinc, Y., Unal, U., and Alaca, B.E., Microelectron. Eng., 2015, vol. 134, pp. 60–67.
Bírlík, I. and Akazem, N.F., J. Sci. Eng., 2018, vol. 20, p. 689.
Saratha, R., Priya, S.V., and Thilagavathy, P., J. Chem., 2009, vol. 6, pp. 785–795.
Muthukrishnan, P., Jeyaprabha, B., and Prakash, P., Int. J. Ind. Chem., 2014, vol. 5, no. 1, 5.
Chuka, C.E., Odio, B.O., Chukwuneke, J.L., and Sinebe, J.E., Int. J. Sci. Technol. Res., 2014, vol. 3, pp. 306–310.
Rose, I. and Whittington, C., Nickel Plating Handbook, Brussels: Nickel Inst., 2014, p. 15.
Vidal, M., Amigo, J.M., Bro, R., Ostra, M., et al., Anal. Methods, 2010, vol. 2, pp. 86–92.
Travers, A. and Muskhelishvili, G., FEBS J., 2015, vol. 282, pp. 2279–2295.
Hu, K., Zhuang, J., Ding, J., Ma, Z., et al., Corros. Sci., 2017, vol. 125, pp. 68–76.
Agboola, O., Achile, F., Fayomi, S.O., Sanni, S.E., et al., J. Bio- Tribo-Corros., 2019, vol. 5, 52. https://doi.org/10.1007/s40735-019-0245-5
Agboola, O., Adedoyin, T., Sanni, S.E., Fayomi, S.O., et al., Anal. Bioanal. Electrochem., 2019, vol. 11, pp. 1304–1328.
Oriňáková, R., Turoňová, A., Kladeková, D., Gálová, M., et al., J. Appl. Electrochem., 2006, vol. 36, pp. 957–972.
Khadom, A.A., Yaro, A.S., Musa, A.Y., Mohamad, A.B., et al., J. Korean Chem. Soc., 2012, vol. 56, pp. 406–415.
Ayawei, N., Angaye, S.S., Wankasi, D., and Dikio, E.D., Open J. Phys. Chem., 2015, vol. 5, pp. 56–70.
Ayawei, N., Ekubo, A.T., Wankasi, D., and Dikio, E.D., Orient. J. Chem., 2015, vol. 31, pp. 1307–1318.
Amin, M.T., Alazba, A.A., and Shafiq, M., Sustainability, 2015, vol. 7, pp. 15302–15318.
Ayawei, N., Ebelegi, A.N., and Wankasi, D., J. Chem., 2017, vol. 2017, 3039817.
Onuchukwu, A.I., Mater. Chem. Phys., 1990, vol. 24, pp. 337–341.
Doche, M.L., Rameau, J.J., Durand, R., and Novel-Cattin, F., Corros. Sci., 1999, vol. 41, pp. 805–826.
Ameh, P.O., Ukoha, P., Ejikeme, P., and Eddy, N.O., Ind. Chem., 2016, vol. 2, 1000119.
Rose, I. and Whittington, C., Nickel Plating Handbook, Brussels: Nickel Inst., 2014.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
The original online version of this article was revised: The name of the seventh author should read Itopa Godwin Akande.
About this article
Cite this article
Oluranti Agboola, Johnson, F., Sanni, S.E. et al. Thermodynamic, Adsorption Isotherms and Electrochemical Investigations of Nickel Electroplating on Mild Steel in Electrolyte Containing Deoxyribonucleic Acid from Citrus aurantium as Additive. Surf. Engin. Appl.Electrochem. 56, 684–696 (2020). https://doi.org/10.3103/S1068375520060022
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S1068375520060022