Abstract
Invasive alien species have environmental, economic and social impacts, disproportionally threatening livelihood and food security of smallholder farmers in low- and medium-income countries. Fall armyworm (FAW) (Spodoptera frugiperda), an invasive insect pest from the Americas, causes considerable losses on maize to smallholder farmers in Africa since 2016. The increased use of pesticides to control FAW in Africa raises concerns for health and environmental risks resulting in a growing interest in research on biological control options for smallholder farmers. In order to evaluate the occurrence of local natural enemies attacking FAW, we collected on a weekly basis FAW eggs and larvae during a maize crop cycle in the rainy season of 2018–2019 at four locations in the Lusaka and Central provinces in Zambia. A total of 4373 larvae and 162 egg masses were collected. For each location and date of collection, crop stage, the number of plants checked and amount of damage were recorded to analyse which factors best explain the occurrence of the natural enemy species on maize. Overall parasitism rates from local natural enemies at each location varied between 8.45% and 33.11%. We identified 12 different egg-larval, larval and larval-pupal parasitoid species. Location, maize growth stage, pest density and larval stage significantly affected parasitoid species occurrence. Our findings indicate that there is potential for increasing local populations of natural enemies of FAW through conservation biological control programmes and develop safe and practical control methods for smallholder farmers.
Similar content being viewed by others
Key message
-
A survey was conducted to identify local parasitoids attacking fall armyworm in Zambia.
-
Factors influencing parasitoid occurrence were analysed.
-
12 parasitoids species have been found attacking fall armyworm.
-
Location, maize growth stage, pest density and larval stage affected parasitoid occurrence.
-
Locally developed biocontrol programmes are needed for safe management of pests in Africa.
Introduction
Invasive alien species (IAS) have environmental, economic and social impacts, disproportionally threatening livelihood and food security of smallholder farmers in low- and medium-income countries (LMIC) (Early et al. 2016; Paini et al. 2016; Pratt et al. 2017). Following a biological invasion, smallholder farmers in LMIC face important crop losses due to a lack of knowledge about management practices at early stages of the invasion (Machekano et al. 2017). Additionally, local natural enemies might not be efficient or adapted in keeping the new IAS under control in the newly invaded ecosystem. A range of methods for control and management of IAS relying on prevention, monitoring and rehabilitation of impacted systems is available through various techniques such as chemical, biological and physical control methods (DiTomaso et al. 2017).
Despite the number of biological control agents commercially available, the efficacy and safety of the products, and the many success stories using various biological control techniques, there is still a lack of uptake in biological control (Barratt et al. 2018; Brodeur et al. 2018; Messing and Brodeur 2018; van Lenteren 2012). In particular, classical biological control through the introduction of exotic natural enemies has managed to save important staple and cash crops from IAS where chemicals have failed including the citrus industry and cassava production (Menzler-Hokkanen 2006). However, investigations on classical biocontrol agents can be long-lasting and, when no suitable agent is available or when time is running out to save crops, augmentative and conservation biological control can play an important role in mitigating damage caused by established IAS. Although augmentative and conservation biological control strategies use local natural enemies to target a pest, their implementation differs regarding the type of biological control agents and techniques used (Lazarovits et al. 2007).
Parasitoids are among the most widely used natural enemies in biological control. Various biotic and abiotic factors such as temperature, rainfall, altitude, landscape characteristics (connectivity, complexity and composition), food and shelter sources, sink habitats, host density, volatile emissions and seasonality can influence the diversity, abundance and parasitism rates in the field (Bernasconi Ockroy et al. 2001; D’Alessandro et al. 2009; Mailafiya et al. 2010; Tscharntke and Brandl 2004). These factors are important to evaluate and understand when searching for potential biological control agents as they will influence the spatial and temporal activities of the natural enemy populations. Hence, evaluating the factors influencing the occurrence and abundance of parasitoids alongside the natural enemy species present is a crucial first step in order to plan successful and practical biological control programmes for smallholder farmers.
The fall armyworm (FAW), Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae), a highly transboundary migratory moth native to the Americas, was first detected in Central and Western Africa in early 2016 (Goergen et al. 2016), and since then it has spread across the African and Asian continents (Rwomushana et al. 2018) causing significant damage to maize, its principal food source. In Zambia, FAW infestation was first detected on maize in late 2016 and was reported to affect 22% of the maize grown for the 2017 crop season (IPPC 2017). In 2018, 98% of farmers reported maize to be affected in Zambia with an average maize loss of 35% (Rwomushana et al. 2018). As maize is the major staple food in Africa, FAW has been identified as a serious threat to food and nutrition security for smallholder farmers if not controlled. When FAW was first detected, the immediate reaction was to use chemical control to contain the spread and to minimise damage (Tambo et al. 2020). However, the excessive use of pesticides has raised concerns not only for health and environmental risks but also for sustainability efforts, undermining integrated pest management strategies for smallholder farmers (Harrison et al. 2019; Sisay et al. 2019).
This study is the first step towards a biological control programme to control fall armyworm for smallholder farmers in Zambia. The aim of this study was to assess which local parasitoid species successfully develop in FAW in Zambia and the factors influencing the occurrence of these local species over a maize crop season.
Materials and methods
Field location
Fieldwork was carried out during the rainy season of December-March 2018–2019. Four sampling sites were planted with maize at the onset of the rain during the 3rd week of December (Fig. 1). The four sites were located in the Lusaka and Central provinces; the Zambia Agriculture Research Institute (ZARI)—Mt Makulu, Chilanga (15,549,023; 28,250,960, altitude: 1229 m), Kasisi Agricultural Training Centre (Kasisi) (15,258,464; 28,477,081, altitude: 1113 m), Golden Valley Agricultural Research Trust, Chisamba (14,967,373; 28,097,464, altitude: 1147 m) and Chalimbana (CABI) (15,359,412; 28,488,572, altitude: 1130 m). Additionally, between September and December 2018 (dry season), larvae were irregularly collected at ZARI and Chisamba on irrigated maize. Standard agronomic practices for maize fields were adopted as recommended by the Ministry of Agriculture to smallholder farmers in Zambia.
Egg mass and larva collections
After maize emergence, each site was visited weekly for collecting FAW eggs and larvae until tasselling and silking stages. The numbers of plants checked were counted using a hand tally counter, and plants were selected randomly in a zigzag (W) pattern until the field was covered. Each plant was assessed thoroughly for egg masses and larvae in a non-destructive manner to allow continuous collection over the crop cycle. Hidden larvae in whorls or between the leaves were collected using a paintbrush to avoid damaging the maize plants. For each collection, date, maize crop stages (Table 1), number of plants showing fresh damage and maize damage level using the Davis scale were recorded (Davis and Williams 1992). The number of plants with fresh damage and the maize damage level were recorded using two zigzag patterns in the field consisting of 5 stations (locations in the field where the assessment starts) of 10 consecutive plants each for a total of 100 plants assessed per site and date combination.
Rearing of samples
On the same day of the field collection, egg masses and larvae collected from the maize fields were placed individually in Petri dishes (90 mm diameter) and were given a sample ID number. Larval stage was estimated for each larva collected based on head capsule width. Spodoptera frugiperda is known to have highly variable larval development, ranging from five to ten instars depending on host plant suitability and climate (Montezano et al. 2019). We did not assess the exact number of instars and their head capsule width at our sites but assumed that they passed through six instars, as this is often the case in favourable host plant and climatic conditions, and used the head capsule widths usually observed in such situations (e.g. Capinera 2008; Montezano et al. 2019).
Larvae were reared on artificial diet [Frontier Scientific Services (Newark, DE, USA), general diet for Lepidoptera F9772, with antibiotics 14% active chlortetracycline] in the laboratory at ZARI (27 ± 2 °C and 50 ± 5% RH) until emergence of the parasitoids or adult moths. Egg masses and larvae were observed every day, and the development of parasitoids or FAW moths was recorded. Dead larvae and pupae were dissected to record absence or presence of parasitoid larvae.
Identification of parasitoids
All adult parasitoids obtained during this study were morphologically identified by one of the authors (MK) using various identification keys and collections of insects gathered during previous studies on S. frugiperda parasitoids (Agboyi et al. 2019, 2020). Some representative specimens were brought to the Natural History Museum in London for comparison with the collection holdings and identified with the assistance of specialists (see Acknowledgements). In addition, adults of some species and sub-samples of parasitoid larvae emerging from FAW larvae but unable to pupate in the Petri dishes were subjected to molecular analyses using the mtDNA barcode gene (mtDNA) cytochrome c oxidase subunit 1 (COI) in order to compare them with existing barcode datasets. To obtain barcodes (around 600 bp of the mitochondrial gene from the samples, we followed the protocols described by Kenis et al. (2019). Sequences obtained in the present study were compared with authenticated sequences available from the Barcoding of Life Data system (BOLD; http://www.boldsystems.org/) (Ratnasingham and Hebert 2013) and additional sequences from the GenBank® data base (http://www.ncbi.nlm.nih.gov/genbank/) (Clarck et al. 2016).
Statistical analysis
Parasitism rate
Parasitism rates (± SE) for each location were calculated using the overall number of parasitized larvae (determined through parasitoid emergence and dissection of dead larvae) divided by the total number of FAW larvae collected, expressed as a percentage.
Parasitoid occurrence
Statistical analysis was performed using R version 3.5.2 (R Core Team 2014), using packages multcomp (Hothorn et al. 2008), nnet (Venables and Ripley 2002), ggplot2 (Wickham 2016), car (Fox and Weisberg 2019) and emmeans (Lenth 2020). A multinomial logistic regression model was used to analyse the occurrence of parasitoid species attacking FAW in Zambia. The parasitoid species found within each FAW was set as the categorical dependent variable, whilst location, larval stage, maize crop stage and FAW density level were used as independent variables. FAW density was calculated based on the total numbers of larvae collected divided by the total number of plants checked for each collection, expressed as FAW larvae/plant. FAW density level was included instead of the damage score (Davis scale) or the infestation rate (% of plant showing fresh damage) because FAW density level was determined to be a more accurate measure of FAW population in the field. An analysis of deviance was then conducted to assess the overall effect of each independent variable followed by a Holm–Bonferroni adjusted pairwise comparison test on all significant results.
Parasitism rate and FAW density
A binomial regression was used to analyse the effect of FAW larval density on parasitism rate. Parasitism rate and FAW densities were calculated for each collection (4 sites and 9 sampling dates, n = 36). Parasitism rate was set as the dependent variable and represents the number of parasitized larvae (determined through parasitoid emergence and dissection of dead larvae) divided by the number of FAW larvae collected, expressed as a percentage. FAW larval density was set as the independent variable and represents the number of FAW larvae divided by the total number of plants checked per collection. To account for the overdispersion identified in the model a quasibinomial distribution was used.
FAW density and maize growth stage
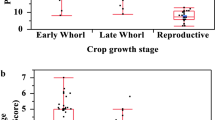
A one-way analysis of variance test was conducted to assess whether there was a statistically significant difference in fall armyworm density across maize development stages. Fall armyworm density is defined as the number of fall armyworm specimens per number of plants checked.
Results
Egg and larvae collection and parasitoid identification
Overall, 4373 larvae and 162 egg masses were collected over the season from the four sites. No egg parasitoid was obtained. From the rainy season, eleven species of larval parasitoids have been recovered from four families (Table 2). From the dry season, one additional parasitoid species recovered from one FAW pupa which was absent during the rainy season has been identified as Metopius discolour Tosquinet. Parasitism rates from local natural enemies at each location were 8.45% ± 0.77 (Chisamba, n = 1313), 12.86% ± 0.99 (Chalimbana, n = 1143), 33.11% ± 1.37 (Kasisi, n = 1172) and 13.15% ± 1.24 (ZARI, n = 745).
A large number of parasitoid larvae did not develop to the adult stage either because they died after emergence from their host larva in the Petri dish without making a cocoon or because the host larva died too early (35.5% of FAW larvae). Sub-samples of the hymenopteran parasitoid larvae that died in the Petri dishes were sent for molecular identification, which revealed that they belonged to the species Chelonus bifoveolatus Szépligeti and Coccygidium luteum Brullé, and further, morphological examinations suggest that both species were abundant. However, it is possible that some larvae that were not sequenced belonged to Chelonus curvimaculatus Cameron, whose larvae are probably similar to those of Ch. bifoveolatus. Because not all larvae could be assigned to Chelonus spp. or C. luteum, dead Hymenoptera larvae were considered as a single parasitoid species in the analyses. Nematodes from the Mermithidae family were also recovered from two FAW but further identification was not possible. Despite that nematodes can be considered as parasitoids, we did not include them in the statistical analysis because insect parasitoids were the focus of the study.
Parasitoid occurrence
Location
The analysis of deviance indicated that location had a significant effect on parasitoid occurrence (d.f. = 36, X2 = 371.57, P < 0.001). The Holm–Bonferroni adjusted pairwise comparison tests indicated that the parasitoid complex at each location where significantly different (P < 0.01). Chisamba had the highest diversity of all sites with nine species of parasitoid found overall including M. discolor from the dry season, whilst ZARI had the lowest diversity with four species. Kasisi had eight parasitoid species present whilst Chalimbana had seven species. Of the species found at all sites, Kasisi had significantly larger numbers of Hymenoptera mortality whilst Chisamba had the greatest proportion of the tachinid Drino quadrizonula (Thomson) (Fig. 2).
Parasitoid species recovered from FAW collected on experimental sites for each location, Zambia. The Holm–Bonferroni adjusted pairwise comparison tests indicated that the parasitoid complex at each location where significantly different (P < 0.01). To account for high mortality in Hymenoptera, occurrence of parasitoid species is visualised as log ((number of parasitoid species/total number of parasitoids) + 1)*100). CBS = Chelonus bifoveolatus, CCC = Chelonus curvimaculatus, CHA = Charops sp., CI = Cotesia icipe, CLB = Coccygidium luteum, DIA = Diadegma sp., DRI = Drino quadrizonula, ECT = Enicospilus capensis, EL = Euplectrus laphygmae, HM = Hymenoptera mortality, PAR = Parapanteles sp., PRI = Pristomerus sp
Maize stage
Occurrence of parasitoid species was significantly impacted by maize crop stage (analysis of deviance test, d.f. = 48, X2 = 162.86, P < 0.001). The Holm–Bonferroni adjusted pairwise comparison tests indicated that the parasitoid complex where significantly different at all maize stages (P < 0.01). The largest number of parasitoid species was found at stages V5-7 with 11 species whilst the highest abundance of parasitoids was found during the V8-10 stages. At stages V11-12 and VT-R1, we found a significant decrease in both occurrence and abundance of parasitoids where the remaining species are D. quadrizonula, C. luteum, C. bifoveolatus and dead hymenopteran larvae (Fig. 3).
Parasitoid species recovered from FAW larvae collected on experimental sites for each maize growth stage, Zambia. The Holm–Bonferroni adjusted pairwise comparison tests indicated that the parasitoid complex where significantly different at all maize stages (P < 0.01). To account for high mortality in Hymenoptera, occurrence of parasitoid species is visualised as log((number of parasitoid species/total number of parasitoids) + 1)*100)
Larval stage
Larval stage had a significant effect on parasitoid occurrence (analysis of deviance test, d.f. = 48, X2 = 404.58, P < 0.001). The Holm–Bonferroni adjusted pairwise comparison tests indicated that the parasitoid complex where significantly different at all larval stages (P < 0.01), except L1-2 and L3 (P = 0.125). Further analysis showed that this difference was largely due to high mortality due to Hymenoptera and D. quadrizonula, with considerable mortality due to Hymenoptera being found for the early larval stages and larger numbers of D. quadrizonula being found in L5 and L6 larvae (Fig. 4). Ten species emerged from the early instar larvae L1 to L4 compared to three species emerging from L5 and L6. The last larval instar was parasitized nearly exclusively by the tachinid fly D. quadrizonula, whereas from L5 larvae we obtained only the tachinid and Hymenoptera larvae belonging to Chelonus spp. and/or Coccygidium luteum.
Parasitoid species recovered from FAW collected on for each larval stage, Zambia. The Holm–Bonferroni adjusted pairwise comparison tests indicated that the parasitoid complex where significantly different at all larval stages (P < 0.01), except L1-2 and L3 (P = 0.125). To account for high mortality in Hymenoptera, occurrence of parasitoid species is visualised as log((number of parasitoid species/total number of parasitoids) + 1)*100)
FAW density
The analysis of deviance indicated that FAW density had a significant effect on parasitoid occurrence (d.f. = 12, X2 = 35.82, P < 0.001). This effect was largely the result of mortality due to Hymenoptera and D. quadrizonula.
Parasitism rate and FAW density
FAW density had a significant effect on parasitism rate (analysis of deviance, d.f. = 1, X2 = 3762.2, P < 0.001). There was a general positive relationship between FAW density and parasitism rate. Most FAW collections over the maize lifecycle resulted in parasitism rates ranging between 0 and 30% when FAW density was between 0.2 and 0.6 larvae per plant. Between 0 and 0.2 larvae per plant, parasitism rates ranged from 0 to 20%. Between 0.6 and 1, parasitism rates ranged from 0 to 45% (Fig. 5).
FAW density and maize growth stage
There was no significant different in fall armyworm density across maize stages (d.f. = 4, F = 0.513, P = 0.738) (Fig. 6). Early larval stage was the most common stage present across maize development, with the exception of V11-V12 where FAW in late larval stage were more frequently found.
Discussion
Eleven species of parasitoids from four families were found attacking eggs (egg-larval parasitoids) and larvae of S. frugiperda during the rainy season 2018–2019 and one additional hymenopteran parasitoid species during the dry season. Our findings are consistent with previous studies on the parasitoid complex of S. frugiperda across Africa. Two of the three most abundant species on our sites, Ch. bifoveolatus and C. luteum, were also the species most frequently collected in Ghana, Benin, Senegal and Tanzania (Agboyi et al. 2020; Koffi et al. 2020; Ngangambe and Mwatawala 2020; Tendeng et al. 2019). Drino quadrizonula, the third abundant species in this study, was found in low numbers on S. frugiperda in Ghana and Benin (Agboyi et al. 2020). Other species found both in Zambia and West Africa, albeit in low numbers, include Cotesia icipe Fernández-Triana & Fiaboe, M. discolor and Charops sp. The two species C. luteum and C. icipe have also been recorded on S. frugiperda in Kenya, Tanzania and Ethiopia and Ch. curvimaculatus in Kenya (Sisay et al. 2018, 2019). However, our study also found five new associations of parasitoids with fall armyworm in Zambia: Euplectrus laphygmae (Ferrière), Parapanteles sp., Diadegma sp., Pristomerus sp. and Enicospilus capensis Thunberg. The large number of parasitoid species having adopted S. frugiperda as host in Africa may be due to the presence of several other Spodoptera species, such as S. exempta, S. exigua and S. littoralis (e.g. Agboyi et al. 2020; Fiaboe et al. 2017; Merrett 1986; Yu et al. 2005), and of Helicoverpa armigera (Van den Berg et al. 1988) which are known hosts of several parasitoids reared during this study.
Overall, larval parasitism rates were rather similar or slightly lower than studies in other parts of Africa. In Ghana, Agboyi et al. (2020) observed average parasitism rates varying from 5.1 to 38.8%, with up to 75% at some sites. In Kenya, Ethiopia and Tanzania, average larval parasitism rates up to 50% were observed (Sisay et al. 2019). Published parasitism rates often vary with sampling methods and calculations (Van Driesche 1983). However, larval parasitism and parasitoid richness of S. frugiperda in Africa are rather high for a species that recently invaded the continent (Cornell and Hawkins 1993). In contrast, while egg parasitism by Telenomus remus was very frequent in Ghana, Benin, Kenya and Tanzania (Agboyi et al. 2020; Sisay et al. 2019), we did not find a single parasitized egg mass in our sites in Zambia. The absence of egg parasitism may be due to a relatively short rainy season compared to other investigated countries, such as Ghana, Benin, Kenya and Tanzania, where bimodal rainfall patterns occur and where S. frugiperda and other insect hosts and their host plants can be found throughout the year (Mailafiya et al. 2010; Van den Berg et al. 1988). In Kenya, a similar study investigated the factors affecting parasitoid diversity, abundance and parasitism of stem borers on maize (Mailafiya et al. 2010). They found the lowest parasitoid richness in the driest locations and highest richness in bimodal rainfall distribution compared to a single rainfall distribution. This suggests that the high parasitoid diversity is maintained through the seasons due to a spatial and temporal continuity of plant and insect hosts. This study is in accordance with our suggestion that the long dry season in Zambia may contribute to the relatively low parasitism rates observed during the short rain season. However, further studies in other years and at other sites and regions in Zambia are needed before drawing firm conclusions regarding the influence of climate on parasitism. In addition, parasitism rates, based on the total number of larvae collected, may be underestimated because host larval mortality was not negligible, and some parasitoid eggs or larvae may have been missed when dead host larvae were dissected. More generally, the laboratory rearing of parasitized larvae should be improved to minimise parasitoid mortality at the larval stage, before pupating.
Our results indicate an effect of location on parasitoid occurrence despite the relatively short distance between each site. Various factors can influence this finding such as variation in rainfall, temperature, landscape, farming and grazing activities. Knowing that habitat fragmentation, intensive tillage, grazing activity, destruction of non-crop habitats and pesticide use can negatively affect parasitoid activities and parasitism rates, these factors need further consideration in the development of biocontrol programmes of FAW. Kasisi, where the highest parasitism rates were observed, is a training centre where conservation and organic agriculture are practiced through the year, while the other sites are located in areas where pesticides are commonly used. Mailafiya et al. (2010) also investigated farming activities in relation to parasitoid diversity and abundance and found that low parasitoid diversity was found where maize is cultivated on a commercial scale and intense grazing activities across seasons. The authors concluded that natural habitat is an important refuge to maintain parasitoid diversity. Refuges may also play an important role in the conservation of natural enemies of FAW (Harrison et al. 2019).
An interesting and unexpected finding among our results was the variation in parasitoid occurrence through the maize growth cycle. The parasitoid species attacking eggs and early larval stages such as Ch. curvimaculatus, C. icipe, E. laphygmae, Parapanteles sp., Diadegma sp. and Pristomerus sp. were observed only during the vegetative maize growth stages (V2-4 to V8-10) despite that all FAW stages (eggs, early and late larval stages) were present until VT-R1, and that no difference in FAW density was found across maize growth stages. These parasitoid species were absent beyond the V8-10 stage. During the last two maize stages both occurrence and abundance of parasitoids recorded from the FAW larvae decrease and mainly the tachinid fly D. quadrinozula remains associated with FAW larvae. A few or a combination of possible factors may influence this effect such as plant structural complexity, composition of vegetation, host quality, host density, volatile organic compounds and seasonality. Plant structural complexity is known to affect host-finding success and foraging behaviour where more hosts are parasitized on simple plants compared to complex plants (Andow and Prokrym 1990; Gingras and Boivin 2002; Gingras et al. 2002, 2003; Lukianchuk and Smith 1997). So far, these studies have mostly involved generalist egg parasitoids of the genus Trichogramma. When maize reaches the reproductive stage, the plant displays a more complex structure than during the vegetative stage with one to twelve leaves. Therefore, as the parasitoids found in Zambia are generalists, or specialised on Spodoptera spp. feeding on various plants (Yu et al. 2005), they might not be adapted to forage and parasitize hosts hidden in maize reproductive structures such as cobs, tassels and silks. However, conclusions cannot be drawn without further studies.
Herbivore-induced plant volatiles (HIPVs) emitted by maize plants that attract natural enemies may also play a role in the temporal distribution of parasitoid species in our study. Maize plants of 10 to 21 days old (3–9 leaf stages) emit induced volatiles when Spodoptera exigua or S. littoralis feed on the young leaves and consequently attract more natural enemies compared to undamaged plants (D’Alessandro et al. 2009; Turlings et al. 1991; Fritzsche-Hoballah and Turlings 2001). While most studies on HIPVs and natural enemies have been conducted under controlled laboratory conditions, some have demonstrated their effects under field environments (Aartsma et al. 2019, 2020; Bernasconi Ockroy et al. 2001; De Lange et al. 2018; Drukker et al. 1995; Poelman et al. 2009; Shimoda et al. 1997). Furthermore, a recent study using 3-leaves stage (9–12 days old) maize plants confirmed that S. frugiperda has lower HIPVs induction on maize than other Spodoptera species (De Lange et al. 2020). More field and laboratory-based studies are needed to understand this tri-trophic interaction between maize, S. frugiperda and its natural enemies.
FAW density also has a significant effect on parasitoid occurrence. A larger number of parasitoids and species were recovered when FAW density peaks at V5-7 and V8-10. S. frugiperda is known to prefer feeding on vegetative stages of maize when the whorl is still present (Capinera 2008), and results from our study show that FAW larvae population builds up until V8-10 and then starts decreasing from V11-12. A recent study conducted under field, field cages and greenhouse conditions aimed at understanding FAW larval choice during maize reproductive stages demonstrated that feeding sites are chosen by first-instar larvae (Pannuti et al. 2016). In addition, leaves from reproductive stages and opened tassels were not suitable for development and survival of early larval stages compared to silk, closed tassels and kernels tissues. Our findings are consistent with this study as we observed FAW larval density declining from V11-12 when leaves start drying up and tassels are developing. During field collection, we have also observed FAW larvae feeding on closed tassels and silk during maize stages VT-R1 but not on opened tassels. According to these observations, maize growth stages and structures available influence FAW larval density which thus affects parasitoid occurrence. As shown in the results, this difference in parasitoid occurrence is mainly due to the tachinid fly D. quadrizonula which is associated with late larval stages which become more abundant later in the crop cycle, from V8-10. As demonstrated above, a tri-trophic approach is needed in future studies to fully understand interactions between maize growth stages, S. frugiperda and its natural enemies.
The relationship found between FAW larval stages and parasitoid species is consistent with literature on similar species attacking FAW in the Americas or from the same species attacking other Noctuidae pests in Africa. Ten of the twelve species found in Zambia have been recovered from larvae collected in the first to fourth larval stages. This finding is encouraging for biological control programmes as early larval stages are the stages that need to be controlled before too much damage is done to the crop. In the Americas, the egg-larval parasitoid Ch. insularis is the most common parasitoid of fall armyworm (González-Maldonado et al. 2014; Jaraleño-Teniente et al. 2020; Meagher et al. 2016; Molina-Ochoa et al. 2003; Ordóñez-García et al. 2015; Ruíz-Nájera et al. 2007). While other countries in Africa have recorded one species of Chelonus (Ch. curvimaculatus in Kenya (Sisay et al. 2018; 2019) and Ch. bifoveolatus in Ghana, Benin, Senegal and Tanzania (Agboyi et al. 2020; Koffi et al. 2020; Ngangambe and Mwatawala 2020; Tendeng et al. 2019), in Zambia we have recorded two species. This could have some implication for interspecific competition, therefore, further studies are needed to understand the interaction and ecological niches of both species, mainly if classical biological control is considered in the future with Ch. insularis. Cotesia marginiventris, a species related to C. icipe, attacks the first to second instar larvae of noctuid moths. In our study, all except one (L3) C. icipe parasitoids were recovered from larvae collected as L1 and L2. This parasitoid was present at low parasitism rates during the early maize crop stages only. Similarly, E. laphygmae was also present at the early stage of the fall armyworm infestation, attacking L1-L2 instar larvae at very low incidence. However, literature reported that E. laphygmae attacks L2–L4 instar larvae of native African Noctuidae pests (Gerling and Limon 1976; Gudeta 1998). The same behaviour was observed from this species in Malawi during an outbreak of S. exempta, where E. laphygmae appeared early in cereal crops followed by Apanteles (= Cotesia) species (Smee 1946). Very limited information is available on the biology of Parapanteles species, but from our results and a study in Africa (Valerio et al. 2005), the genus seems to prefer L1-L2 for oviposition.
Coccygidium luteum, one of the most common parasitoids on FAW in Africa, was recovered from larvae collected as L1–L4 in the field. A study by Agboyi et al. (2019) showed that C. luteum females attack the L1 stage of FAW, but that the parasitoid larvae emerge from the host between 8 and 10 days after oviposition in L1, which correspond to the development time of unparasitized FAW larvae up to L4 (Montezano et al. 2019). The only tachinid fly recovered from fall armyworm larvae was Drino quadrinozula. Tachinid flies are known to attack mostly late instar larvae (Van den Berg et al. 1988). Over the crop cycle, they started to appear only when the population of late instar larvae had peaked at V8-10 maize stage. This category of parasitoids can be of importance in the control of FAW mainly by reducing subsequent dispersal of adult moths to other fields. While the rearing of tachinid flies can be difficult in the laboratory, further research on conservation biocontrol to improve parasitism and population would benefit the fight against FAW.
Pristomerus spinator is found in the Americas to attack FAW larval stage L3-4 (Cave 1995) and on the African continent, Pristomerus spp. have been recorded attacking L2-4 of Helicoverpa armigera (Van den Berg et al. 1988) which is in accordance with our results. Van den Berg et al. (1988) have reviewed in detail the natural enemies of H. armigera in Africa of which many species or genera overlap with the species found in this study. As H. armigera has similar larval development stages as S. frugiperda (L1-6), we can assume that the following parasitoids attack similar host stages: Charops sp. (L1), Diadegma sp. (L3), Enicospilus sp. (L3-4) and Metopius discolor (L5-6), which again is consistent with our findings. Only one or two specimens of these latter parasitoids were recorded during the 2018–2019 season, and the same species are also observed to be rare on H. armigera except for Charops sp. However, the FAW larval stages associated with the parasitoid species in our study are the stages at the day of field collection, therefore, we cannot be sure of the host stage attacked.
Numerous studies looked into the relation between parasitism and host density (May et al. 1981; Stirling 1987; Walde and Murdoch 1988). This relation can be categorised as direct, inverse or independent (Godfray 1994). Although our results show a general direct relationship between FAW density and parasitism rate, this result needs to be considered with caution because we did not discriminate between spatial (within-generation) and temporal (between-generation) density dependence, which are very different concepts (Dempster and Pollard 1986). In our study, we neither sampled enough sites nor did we study enough generations to draw firm conclusions on density dependence. In addition, host-parasitoid density should better be assessed per parasitoid species, which was not possible here because most parasitoids died as undetermined Hymenoptera, and some species only had one or two specimens over the whole season.
In our study, twelve species were found to attack and develop on FAW egg, larval and pupal stages. Nevertheless, none of the species seems to show high efficacy in the field to keep FAW under control. The biological features of highly mobile pests and their shifts in crops and habitats make them very successful in invading a new environment. However, their natural enemies may encounter difficulties adapting to new habitats in order to follow the seasonal movement of the pest which eventually impacts their effectiveness as biological control agents (Hirose 1998). In conservation biological control, effective natural enemies of mobile pests should be characterised by good colonisation ability, dispersal of a portion of the new generation from the fields when hosts are present, high mobility and have a shorter generation time than the host. In the light of these recommendations, many more studies will be critical for evaluating the biology and ecology of local African parasitoids which are still unknown and poorly studied for many. Furthermore, surveys of natural enemies occurring sporadically at various stages of the crop cycle may lead to an underestimation of the parasitoid complex and various parasitoid species might be overlooked in surveys. As conservation biological control focuses, among other strategies, on enhancing food and shelter resources to local natural enemies, a thorough inventory of the natural enemies and their roles during the crop cycle are critical to assess timely and effective conservation practices.
In conclusion, our study found associations of local parasitoid species with the invasive alien species S. frugiperda in Zambia. We also demonstrated that location, maize growth stage, pest density and larval stage are factors strongly influencing parasitoid species occurrence. This research showed the importance of understanding the spaciotemporal activities of local natural enemies and tri-trophic interactions when elaborating a biological control programme. This is specifically relevant for conservation biological control in order to develop timely management practices against a specific pest stage in order to enhance parasitoid populations and mobility across crop and non-crop habitats. Further studies are needed to determine the exact species of parasitoids through both molecular and morphological identification, the effect of maize growth stage on parasitoid attractiveness, abiotic factors influencing diversity and abundance, the population dynamics of the main parasitoids and the role of HIPVs on parasitism of fall armyworm in order to develop safe and practical control methods for smallholder farmers.
Author contributions
LDG and MK conceived and planned the experiments. LDG, TM and MM collected the field and laboratory data. G. Chipabika helped supervise the overall project in Zambia. AB, G. Cafà and LO performed the molecular identification of the parasitoid larvae. LDG, AL and KR contributed to the analysis and interpretation of the results. LDG took the lead in writing the manuscript with support from MK and MD, and all authors provided critical feedback and helped shape the research, analysis and manuscript.
Code availability
Not applicable.
Change history
22 September 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10340-021-01435-7
References
Aartsma Y, Hao Y, Dicke M, van der Werf W, Poelman EH, Bianchi FJ (2020) Variation in parasitoid attraction to herbivore-infested plants and alternative host plant cover mediate tritrophic interactions at the landscape scale. Landsc Ecol 35:907–919. https://doi.org/10.1007/s10980-020-00988-9
Aartsma Y, Leroy B, Van der Werf W, Dicke M, Poelman EH, Bianchi FJJA (2019) Intraspecific variation in herbivore-induced plant volatiles influences the spatial range of plant-parasitoid interactions. Oikos 128:77–86. https://doi.org/10.1111/oik.05151
Agboyi LK et al (2019) Evidence of leaf consumption rate decrease in fall armyworm, Spodoptera frugiperda Larvae Parasitized by Coccygidium luteum. Insects 10:410. https://doi.org/10.3390/insects10110410
Agboyi LK, Goergen G, Beseh P, Mensah SA, Clottey VA, Glikpo R, Buddie A, Cafà G, Offord L, Day R, Rwomushana I, Kenis M (2020) Parasitoid complex of fall armyworm, Spodoptera frugiperda, in Ghana and Benin. Insects 11:68. https://doi.org/10.3390/insects11020068
Andow DA, Prokrym DR (1990) Plant structural complexity and host-finding by a parasitoid. Oecologia 82:162–165. https://doi.org/10.1007/BF00323530
Barratt BIP, Moran VC, Bigler F, Van Lenteren JC (2018) The status of biological control and recommendations for improving uptake for the future. Biocontrol 63:155–167. https://doi.org/10.1007/s10526-017-9831-y
Bernasconi Ockroy ML, Turlings TC, Edwards PJ, Fritzsche-Hoballah ME, Ambrosetti L, Bassetti P, Dorn S (2001) Response of natural populations of predators and parasitoids to artificially induced volatile emissions in maize plants (Zea mays L.). Agr Forest Entomol 3:201–209. https://doi.org/10.1046/j.1461-9555.2001.00107.x
Brodeur J, Abram PK, Heimpel GE, Messing RH (2018) Trends in biological control: public interest, international networking and research direction. Biocontrol 63:11–26. https://doi.org/10.1007/s10526-017-9850-8
Capinera JL (2008) Fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). In: Capinera JL (ed) Encyclopedia of Entomology. Springer, The Netherlands, pp 1409–1412
Cave RD (1995) Parasitoides de Plagas Agrícolas en América Central. Zamorano Academic Press, Zamorano, Honduras
Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2016) GenBank. Nucleic Acids Res. 2016, 44 (database issue), D67–D72. https://doi.org/10.1093/nar/gkx1094
Cornell HV, Hawkins BA (1993) Accumulation of native parasitoid species on introduced herbivores: a comparison of hosts as natives and hosts as invaders. Am Nat 141:847–865. https://doi.org/10.1086/285512
Davis FM, Williams WP (1992) Visual rating scales for screening whorl-stage corn for resistance to fall armyworm. Tech. Bull. 186, Mississippi State University, USA
De Lange ES et al (2020) Spodoptera frugiperda caterpillars suppress herbivore-induced volatile emissions in maize. J Chem Ecol 46:344–360. https://doi.org/10.1007/s10886-020-01153-x
De Lange ES, Farnier K, Degen T, Gaudillat B, Aguilar-Romero R, Bahena-Juárez F, Oyama K, Turlings TC (2018) Parasitic wasps can reduce mortality of teosinte plants infested with fall armyworm: support for a defensive function of herbivore-induced plant volatiles. Front Ecol Evol 6:55. https://doi.org/10.3389/fevo.2018.00055
Dempster JP, Pollard E (1986) Spatial heterogeneity, stochasticity and the detection of density dependence in animal populations. Oikos 46:413–416. https://doi.org/10.2307/3565842
DiTomaso JM, Van Steenwyk RA, Nowierski RM, Vollmer JL, Lane E, Chilton E, Burch PL, Cowan PE, Zimmerman K, Dionigi CP (2017) Enhancing the effectiveness of biological control programs of invasive species through a more comprehensive pest management approach. Pest Manag Sci 73:9–13. https://doi.org/10.1002/ps.4347
Drukker B, Scutareanu P, Sabelis MW (1995) Do anthocorid predators respond to synomones from Psylla-infested pear trees under field conditions? Entomol Exp Appl 77:193–203. https://doi.org/10.1111/j.1570-7458.1995.tb02001.x
D’Alessandro M, Brunner V, von Mérey G, Turlings TC (2009) Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J Chem Ecol 35:999. https://doi.org/10.1007/s10886-009-9692-7
Early R, Bradley BA, Dukes JS, Lawler JJ, Olden JD, Blumenthal DM, Gonzalez P, Grosholz ED, Ibañez I, Miller LP, Sorte CJB, Tatem AJ (2016) Global threats from invasive alien species in the twenty-first century and national response capacities. Nat Commun 7:12485. https://doi.org/10.1038/ncomms12485
Fiaboe KK, Fernández-Triana J, Nyamu FW, Agbodzavu KM (2017) Cotesia icipe sp. n., a new Microgastrinae wasp (Hymenoptera, Braconidae) of importance in the biological control of Lepidopteran pests in Africa. J Hymenopt Res 61:49–64. https://doi.org/10.3897/jhr.61.21015
Fox J, Weisberg S (2019) An R companion to applied regression, Third edition. Sage, Thousand Oaks CA. https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Fritzsche-Hoballah MEF, Turlings TCJ (2001) Experimental evidence that plants under caterpillar attack may benefit from attracting parasitoids. Evol Ecol Res 3:553–565
Gerling D, Limon S (1976) A biological review of the genus Euplectrus [Hym.: Eulophidae] with special emphasis on E. Laphygmae as a parasite of Spodoptera littoralis [Lep.: Noctuidae]. Entomophaga 21:179–187
Gingras D, Boivin G (2002) Effect of plant structure, host density and foraging duration on host finding by Trichogramma evanescens (Hymenoptera: Trichogrammatidae). Environ Entomol 31:1153–1157. https://doi.org/10.1603/0046-225X-31.6.1153
Gingras D, Dutilleul P, Boivin G (2002) Modeling the impact of plant structure on host-finding behavior of parasitoids. Oecologia 130:396–402. https://doi.org/10.1007/s00442-001-0819-y
Gingras D, Dutilleul P, Boivin G (2003) Effect of plant structure on host finding capacity of lepidopterous pests of crucifers by two Trichogramma parasitoids. Biol Control 27:25–31. https://doi.org/10.1016/S1049-9644(02)00189-5
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton University Press, Princeton
Goergen G, Kumar PL, Sankung SB, Togola A, Tamò M (2016) First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 11:10. https://doi.org/10.1371/journal.pone.0165632
González-Maldonado MB, García-Gutiérrez C, González-Hernández A (2014) Parasitismo y distribución de Campoletis sonorensis Cameron (Hymenoptera: Ichneumonidae) y Chelonus insularis Cresson (Hymenoptera: Braconidae), parasitoides del gusano cogollero en maíz en Durango, México. Vedalia 15:47–53
Gudeta S (1998) Euplectrus laphygmae as a potential biological control agent in Eastern Ethiopia. Pest Manag J Ethiop 2:66–70
Gurr GM, Wratten SD, Altieri MA (2004) Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods. Collingwood, Australia and Wallingford, UK: CSIRO (Australia) and CABI (Europe)
Harrison RD, Thierfelder C, Baudron F, Chinwada P, Midega C, Schaffner U, Van Den Berg J (2019) Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: providing low-cost, smallholder friendly solutions to an invasive pest. J Environ Manag 243:318–330. https://doi.org/10.1016/j.jenvman.2019.05.011
Hirose Y (1998) Conservation biological control of mobile pests: problems and tactics. In: Barbosa P (ed) Conservation biological control. Academic Press, Cambridge, pp 221–233
Hothorn T, Bretz F, Westfall P (2008) Simultaneous Inference in general parametric models. Biometrical J 50:346–363. https://doi.org/10.1002/bimj.200810425
IPPC (2017) IPPC official pest report, (No. ZMB-02/2). Rome, Italy, FAO. https://www.ippc.int/
Jaraleño-Teniente J, Lomeli-Flores JR, Rodríguez-Leyva E, Bujanos-Muñiz R, Rodríguez-Rodríguez SE (2020) Egg Parasitoids Survey of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) in Maize and Sorghum in Central Mexico. Insects 11:157. https://doi.org/10.3390/insects11030157
Kenis M et al (2019) Telenomus remus, a candidate parasitoid for the biological control of Spodoptera frugiperda in Africa, is already present on the continent. Insects 10:92. https://doi.org/10.3390/insects10040092
Koffi D, Kyerematen R, Eziah VY, Agboka K, Adom M, Goergen G, Meagher RL Jr (2020) Natural enemies of the fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Ghana. Fla Entomol 103:85–90. https://doi.org/10.1653/024.103.0414
Lazarovits G, Goettel MS, Vincent C (2007) Adventures in biocontrol. In: Lazarovits G (ed) Vincent C, Goettel MS. Biological control a global perspective. CAB International, Wallingford, pp 1–6
Lenth R (2020) Emmeans: estimated marginal means, aka least-squares means. R package version 1.4.5. https://CRAN.R-project.org/package=emmeans
Lukianchuk JL, Smith SM (1997) Influence of plant structural complexity on the foraging success of Trichogramma minutum: a comparison of search on artificial and foliage models. Entomol Exp Appl 84:221–228. https://doi.org/10.1046/j.1570-7458.1997.00219.x
Machekano H, Mvumi BM, Nyamukondiwa C (2017) Diamondback moth Plutella xylostella (L.) in Southern Africa: research trends, challenges and insights on sustainable management options. Sustainability 9:91. https://doi.org/10.3390/su9020091
Mailafiya DM, Le Ru BP, Kairu EW, Calatayud PA, Dupas S (2010) Factors affecting stem borer parasitoid species diversity and parasitism in cultivated and natural habitats. Environ Entomol 39:57–67. https://doi.org/10.1603/EN09196
May RM, Hassell MP, Anderson RM, Tonkyn DW (1981) Density dependence in host-parasitoid models. J Anim Ecol. https://doi.org/10.2307/4142
Meagher RL Jr, Nuessly GS, Nagoshi RN, Hay-Roe MM (2016) Parasitoids attacking fall armyworm (Lepidoptera: Noctuidae) in sweet corn habitats. Biol Control 95:66–72. https://doi.org/10.1016/j.biocontrol.2016.01.006
Menzler-Hokkanen I (2006) Socioeconomic significance of biological control. In: Eilenberg J, Hokkanen HM (eds) An ecological and societal approach to biological control. Springer, Dordrecht, pp 13–25
Merrett PJ (1986) Natural enemies of the African armyworm, Spodoptera exempta (Walker) (Lepidoptera: Noctuidae), in Tanzania. B Entomol Res 76:545–552. https://doi.org/10.1017/S0007485300015054
Messing R, Brodeur J (2018) Current challenges to the implementation of classical biological control. Biocontrol 63:1–9. https://doi.org/10.1007/s10526-017-9862-4
Molina-Ochoa J, Carpenter JE, Heinrichs EA, Foster JE (2003) Parasitoids and parasites of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean Basin: an inventory. Fla Entomol 86:254–289. https://doi.org/10.1653/0015-4040(2003)086[0254:PAPOSF]2.0.CO;2
Montezano DG, Specht A, Sosa-Gómez DR, Roque-Specht VF, de Paula-Moraes SV, Peterson JA, Hunt TE (2019) Developmental parameters of Spodoptera frugiperda (Lepidoptera: Noctuidae) immature stages under controlled and standardized conditions. J Agr Sci 11:76–89. https://doi.org/10.5539/jas.v11n8p76
Ngangambe MH, Mwatawala MW (2020) Effects of entomopathogenic fungi (EPFs) and cropping systems on parasitoids of fall armyworm (Spodoptera frugiperda) on maize in eastern central, Tanzania. Biocontrol Sci Techn 30:418–430. https://doi.org/10.1080/09583157.2020.1726878
Ordóñez-García M, Rios-Velasco C, Berlanga-Reyes DI, Acosta-Muñiz CH, Salas-Marina MÁ, Cambero-Campos OJ (2015) Occurrence of natural enemies of Spodoptera frugiperda (Lepidoptera: Noctuidae) in Chihuahua, Mexico. Fla Entomol 98:843–847. https://doi.org/10.2307/24587732
Paini DR, Sheppard WA, Cook DC, de Barro PJ, Worner SP, Thomas MB (2016) Global threat to agriculture from invasive species. Proc Natl Acad Sci USA 113:7575–7579. https://doi.org/10.1073/pnas.1602205113
Pannuti LER, Baldin ELL, Hunt TE, Paula-Moraes SV (2016) On-plant larval movement and feeding behavior of fall armyworm (Lepidoptera: Noctuidae) on reproductive corn stages. Environ Entomol 45:192–200. https://doi.org/10.1093/ee/nvv159
Poelman EH, Oduor A, Broekgaarden C, Hordijk CA, Janssen JJ, van Loon JJA, van Dam NM, Vet LEM, Dicke M (2009) Field parasitism rates of caterpillars on Brassica oleracea plants are reliably predicted by differential attraction of Cotesia parasitoids. Funct Ecol 23:951–962. https://doi.org/10.1111/j.1365-2435.2009.01570.x
Pratt CF, Constantine KL, Murphy ST (2017) Economic impacts of invasive alien species on African smallholder livelihoods. Glob Food Secur 14:31–37. https://doi.org/10.1016/j.gfs.2017.01.011
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ratnasingham S, Hebert PDN (2013) A DNA-based registry for all animal species: the barcode index number (BIN) system. PLoS ONE 8:e66213. https://doi.org/10.1371/journal.pone.0066213
Ruız-Nájera RE, Molina-Ochoa J, Carpenter JE, Espinosa-Moreno JA, Ruız-Nájera JA, Lezama-Gutiérrez R, Foster JE (2007) Survey for hymenopteran and dipteran parasitoids of the fall armyworm (Lepidoptera: Noctuidae) in Chiapas, Mexico. J Agric Urban Entomol 24:35–42. https://doi.org/10.3954/1523-5475-24.1.35
Rwomushana I, Bateman M, Beale T, Beseh P, Cameron K, Chiluba M et al (2018) Fall armyworm: Impacts and implication for Africa. Evidence Note Update, CAB International, Wallingford
Schmidt MH, Thewes U, Thies C, Tscharntke T (2004) Aphid suppression by natural enemies in mulched cereals. Entomol Experim Appl 113:87–93. https://doi.org/10.1111/j.0013-8703.2004.00205.x
Shimoda T, Takabayashi J, Ashihara W, Takafuji A (1997) Response of predatory insect Scolothrips takahashii toward herbivore-induced plant volatiles under laboratory and field conditions. J Chem Ecol 23:2033–2048. https://doi.org/10.1023/B:JOEC.0000006487.49221.df
Sisay B, Simiyu J, Malusi P, Likhayo P, Mendesil E, Elibariki N, Wakgari M, Ayalew G, Tefera T (2018) First report of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), natural enemies from Africa. J Appl Entomol 142:800–804. https://doi.org/10.1111/jen.12534
Sisay B, Simiyu J, Mendesil E, Likhayo P, Ayalew G, Mohamed S, Subramanian S, Tefera T (2019) Fall armyworm, Spodoptera frugiperda infestations in East Africa: assessment of damage and parasitism. Insects 10:195. https://doi.org/10.3390/insects10070195
Smee C (1946) Report of Entomologist 1945. Zomba Department of Agriculture, Nyasaland, p 5
Stirling PD (1987) The frequency of density dependence in insect host-parasitoid systems. Ecology 68:844–856. https://doi.org/10.2307/1938356
Tambo JA, Kansiime MK, Mugambi I, Rwomushana I, Kenis M, Day RK, Lamontagne-Godwin J (2020) Understanding smallholders’ responses to fall armyworm invasion: cross-country evidence from sub-Saharan Africa. Sci Total Environ 740:140015. https://doi.org/10.1016/j.scitotenv.2020.140015
Tendeng E, Labou B, Diatte M, Djiba S, Diarra K (2019) The fall armyworm Spodoptera frugiperda (JE Smith), a new pest of maize in Africa: biology and first native natural enemies detected. Int J Biol and Chem Sci 13:1011–1026. https://doi.org/10.4314/ijbcs.v13i2.35
Tscharntke T et al (2007) Conservation biological control and enemy diversity on a landscape scale. Biol Control 43:294–309
Tscharntke T, Brandl R (2004) Plant-insect interactions in fragmented landscapes. Annu Rev Entomol 49:405–430. https://doi.org/10.1146/annurev.ento.49.061802.123339
Turlings TCJ, Tumlinson JH, Heath RP, Proveaux AT, Doolittle RE (1991) Isolation and identification of allelochemicals that attract the larval parasitoid, Cotesia marginiventris (Cresson), to the microhabitat of one of its hosts. J Chem Ecol 17:2235–2251. https://doi.org/10.1007/BF00988004
Valerio AA, Whitfield JB, Kole M (2005) Parapanteles, n. sp. (Hymenoptera: Braconidae Microgastrinae) the first record of the genus from the African continent. Zootaxa 855:1–8. https://doi.org/10.11646/zootaxa.855.1.1
Van den Berg H, Waage JK, Cock MJW (1988) Natural enemies of Helicoverpa armigera in Africa-a review. CAB International
Van Driesche RG (1983) Meaning of “percent parasitism” in studies of insect parasitoids. Environ Entomol 12:1611–1622. https://doi.org/10.1093/ee/12.6.1611
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20. https://doi.org/10.1007/s10526-011-9395-1
Venables WN, Ripley BD (2002) Modern applied statistics with S, Fourth edition. Springer, New York. ISBN 0–387–95457–0, http://www.stats.ox.ac.uk/pub/MASS4
Walde SJ, Murdoch WW (1988) Spatial density dependence in parasitoids. Annu Rev Entomol 33:441–466
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag New York. ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org
Yu DS, Van Achterberg K, Horstmann K (2005) World Ichneumonoidea 2004—taxonomy, biology, morphology and distribution. CD/DVD. Taxapad, Vancouver. British Columbia, Canada
Acknowledgements
The authors would like to thanks Andrew Polaszek, Nigel Wyatt and Gavin Broad from the Natural History Museum of London for their support in the identification of some parasitoids. We also want to thanks all ZARI and CABI Southern Africa colleagues for their continuous support with field and laboratory work, as well colleagues from IITA and ICRAF in Zambia for providing feedback on the results during meetings held in Zambia. The authors are grateful to the anonymous reviewers who provided valuable feedback to improve this manuscript.
Funding
The study was conducted as part of the Action on Invasives (AoI) programme funded by the United Kingdom (Department for International Development) and the Netherlands (Directorate-General for International Cooperation). CABI is an international intergovernmental organisation, and gratefully acknowledges the core financial support from our member countries (and lead organisations) including the United Kingdom (Department for International Development), China (Chinese Ministry of Agriculture), Australia (Australian Centre for International Agricultural Research), Canada (Agriculture and Agri-Food Canada), the Netherlands (Directorate-General for International Cooperation), Switzerland (Swiss Agency for Development and Cooperation), and Ireland (Irish Aid, International Fund for Agricultural Development-IFAD). See http://www.cabi.org/about-cabi/who-we-work-with/key-donors/ for full details.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by Donald Weber.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Durocher-Granger, L., Mfune, T., Musesha, M. et al. Factors influencing the occurrence of fall armyworm parasitoids in Zambia. J Pest Sci 94, 1133–1146 (2021). https://doi.org/10.1007/s10340-020-01320-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-020-01320-9