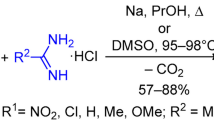
Abstract
During the synthesis of new structural analogs of isothiobarbamine, the amidoalkylation of 6-hydroxy-5-isopropyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one was found to give a mixture of mono-, di-, and trisubstituted products. Possible explanations for low regioselectivity of the reaction are given.
Similar content being viewed by others
References
Pat. RF 552789; 1993 (in Russian).
M. B. Plotnikov, T. M. Plotnikova, T. V. Yakimova, A. S. Saratikov, Bull. Exp. Biol. Med., 1988, 106, 1720; DOI: https://doi.org/10.1007/BF00802177.
M. B. Plotnikov, T. M. Plotnikova, T. V. Yakimova, Z. V. Korobsova, G. A. Chernyshova, A. S. Saratikov, Farmakologiya Toksikologiya [Pharmacology Toxicology], 1988, 51, 38 (in Russian).
P. P. Denisenko, E. Yu. Poltavchenko, Bull. Exp. Biol. Med., 1985, 99, 443; DOI: https://doi.org/10.1007/BF00842734.
S. G. Soboleva, I. F. Gerasimenko, L. G. Kravchuk, L. I. Elfimova, A. A. Dvorkin Yu. A. Simonov, S. A. Andronati, Dokl. AN [Dokl. Chem.], 1992, 327, 349 (in Russian).
B. V. Golomolzin, E. A. Tarakhtii, I. P. Tregubenko, N. M. Perova, Pharm. Chem. J., 1985, 18, 621; DOI: https://doi.org/10.1007/BF00773821.
N. F. Albertson, R. O. Clinton, J. Am. Chem. Soc., 1945, 67, 1222; DOI: https://doi.org/10.1021/ja01223a501.
R. O. Clinton, U. J. Salvador, S. C. Laskowski, C. M. Suter, J. Am. Chem. Soc., 1948, 70, 950; DOI: https://doi.org/10.1021/ja01183a018.
M. I. Attia, A. L. Kansoh, N. R. El-Brollosy, Monatsh. Chem., 2014, 145, 1825; DOI: https://doi.org/10.1007/s00706-014-1253-2.
M. I. Attia, A. A. El-Emam, A. A. Al-Turkistani, A. L. Kansoh, N. R. El-Brollosy, Molecules, 2014, 19, 279; DOI: https://doi.org/10.3390/molecules19010279.
D. G. Doherty, W. T. Burnett, Proc. Soc. Exp. Biol. Med., 1955, 89, 312; DOI: https://doi.org/10.3181/00379727-89-21795.
A. Chose, M. N. Srinivasan, J. Radiat. Res., 1980, 21, 197; DOI: https://doi.org/10.1269/jrr.21.197.
E. G. Valles, C. R. de Castro, J. A. Castro, Toxicology, 1994, 90, 71; DOI: https://doi.org/10.1016/0300-483X(94)90206-2.
A. S. Burke, L. A. MacMillan-Crow, J. A. Hinson, Chem. Res. Toxicol., 2010, 23, 1286; DOI: https://doi.org/10.1021/tx1001755.
M. Kathmann, E. Schlicker, M. Detzner, H. Timmerman, Naunyn-Schmiedeberg’s Arch. Pharmacol., 1993, 348, 498; DOI: https://doi.org/10.1007/BF00173209.
T. Hino, K. Tanaami, K. Yamada, S. Akaboshi, Chem. Pharm. Bull., 1966, 14, 1193; DOI: https://doi.org/10.1248/cpb.14.1193.
G. Schneider, W. Neidhart, T. Giller, G. Schmid, Angew. Chem., Int. Ed., 1999, 38, 2894; DOI: https://doi.org/10.1002/(SICI)1521-3773(19991004)38:19<2894::AID-ANIE2894>3.0.CO;2-F.
Pat. RF 2061686 (in Russian).
S. B. Seredenin, M. V. Voronin, Eksp. Klin. Farm. [Exp. Clin. Pharm.], 2009, No. 1, 3 (in Russian).
L. Monti, G. Franchi, Gazz. Chim. Ital., 1951, 81, 191.
I. A. Novakov, D. S. Sheikin, V. V. Chapurkin, M. B. Navrotskii, A. S. Babushkin, E. A. Ruchko, A. Yu. Maryshev, D. Schols, Russ. J. Gen. Chem., 2020, 90, 352; DOI: https://doi.org/10.1134/S1070363220030056.
I. A. Novakov, D. S. Sheikin, V. V. Chapurkin, M. B. Navrotskii, A. S. Babushkin, Ya. P. Kuznetsov, E. A. Ruchko, V. V. Kachala, A. Yu. Maryshev, D. Schols, Chem. Heterocycl. Compd., 2020, 56, 67; DOI: https://doi.org/10.1007/s10593-020-02624-5.
D. A. Ibrahim, D. S. Lasheen, M. Y. Zaky, A. W. Ibrahim, D. Vullo, M. Ceruso, C. T. Supuran, D. A. Abou El Ella, Bioorg. Med. Chem., 2015, 23, 4989.
K. N. Lyssenko, A. N. Geisman, A. A. Ozerov, M. S. Novikov, Volgogr. Nauchn.-Med. Zh. [Volgogr. J. Med. Research], 2016, 4, 18 (in Russian).
A. A. Ozerov, M. S. Novikov, G. N. Solodunova, M. P. Paramonova, Volgogr. Nauchn.-Med. Zh. [Volgogr. J. Med. Research], 2010, 4, 17 (in Russian).
M. Yu, X. Liu, Z. Li, S. Liu, C. Pannecouque, E. De Clercq, Bioorg. Med. Chem., 2009, 17, 7749; DOI: https://doi.org/10.1016/j.bmc.2009.09.035.
M. Yu, A. Liu, G. Du, L. Naesens, E. Vanderlinden, E. De Clercq, X. Liu, Chem. Biol. Drug. Des., 2011, 78, 596; DOI: https://doi.org/10.1111/j.1747-0285.2011.01180.x.
M. B. Navrotskii, Pharm. Chem. J., 2005, 39, 466; DOI: https://doi.org/10.1007/s11094-006-0002-1.
I. A. Novakov, M. B. Navrotskii, E. K. Zakharova, L. L. Brunilina, Russ. Chem. Bull., 2015, 64, 2545; DOI: https://doi.org/10.1007/s11172-015-1190-1.
I. A. Novakov, M. Artico, A. Mai, D. Rotili, B.S. Orlinson, L. L. Brunilina, M. B. Nawrozkij, Russ. Chem. Bull., 2012, 61, 1399; DOI: https://doi.org/10.1007/s11172-012-0182-7.
D. Murugesan, P. C. Ray, T. Bayliss, G. A. Prosser, J. R. Harrison, K. Green, C. Soares de Melo, T. Feng, L. J. Street, K. Chibale, D. F. Warner, V. Mizrahi, O. Epemolu, P. Scullion, L. Ellis, J. Riley, Y. Shishikura, L. Ferguson, M. Osuna-Cabello, K. D. Read, S. R. Green, D. A. Lamprecht, P. M. Finin, A. J. C. Steyn, T. R. Ioerger, J. Sacchettini, K. Y. Rhee, K. Arora, C. E. Barry, P. G. Wyatt, H. Ingrid, M. Boshoff, ACS Infect. Dis., 2018, 4, 954; DOI: https://doi.org/10.1021/acsinfecdis.7b00275.
L. F. Tietze, T. Eicher, Reactionen und Synthesen im organischchemischen Praktikum und Forschungslaboratorium, Wiley VCH, Weinheim, 1991.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences A. M. Muzafarov on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
Novakov, I.A., Sheikin, D.S., Chapurkin, V.V. et al. Specific features of amidoalkylation of 6-hydroxy-5-isopropyl-2-thioxo-2,3-dihydropyrimidin-4(1H)-one. Russ Chem Bull 69, 2363–2369 (2020). https://doi.org/10.1007/s11172-020-3029-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-020-3029-7