Abstract
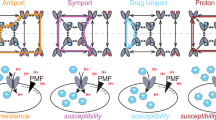
Eukaryotic cells rely on multiple mechanisms to protect themselves from exogenous toxic compounds. For instance, cells can limit penetration of toxic molecules through the plasma membrane or sequester them within the specialized compartments. Plasma membrane transporters with broad substrate specificity confer multiple drug resistance (MDR) to cells. These transporters efflux toxic compounds at the cost of ATP hydrolysis (ABC-transporters) or proton influx (MFS-transporters). In our review, we discuss the possible costs of having an active drug-efflux system using yeast cells as an example. The pleiotropic drug resistance (PDR) subfamily ABC-transporters are known to constitutively hydrolyze ATP even without any substrate stimulation or transport across the membrane. Besides, some MDR-transporters have flippase activity allowing transport of lipids from inner to outer lipid layer of the plasma membrane. Thus, excessive activity of MDR-transporters can adversely affect plasma membrane properties. Moreover, broad substrate specificity of ABC-transporters also suggests the possibility of unintentional efflux of some natural metabolic intermediates from the cells. Furthermore, in some microorganisms, transport of quorum-sensing factors is mediated by MDR transporters; thus, overexpression of the transporters can also disturb cell-to-cell communications. As a result, under normal conditions, cells keep MDR-transporter genes repressed and activate them only upon exposure to stresses. We speculate that exploiting limitations of the drug-efflux system is a promising strategy to counteract MDR in pathogenic fungi.


Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- QS:
-
quorum sensing
- MDR:
-
multiple drug resistance
- MFS:
-
major facilitator superfamily
- PDR:
-
pleiotropic drug resistance
References
Aas, P. A., Otterlei, M., Falnes, P. O., Vågbø, C. B., Skorpen, F., Akbari, M., et al. (2003) Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA, Nature, 421, 859-863, https://doi.org/10.1038/nature01363.
Chernova, T. A., Wilkinson, K. D., and Chernoff, Y. O. (2017) Prions, chaperones, and proteostasis in yeast, Cold Spring Harb. Perspect. Biol., 9, a023663, https://doi.org/10.1101/cshperspect.a023663.
Meunier, J. (2015) Social immunity and the evolution of group living in insects, Philos. Trans. R Soc. Lond. B Biol. Sci., 370, https://doi.org/10.1098/rstb.2014.0102.
Keller, N. P., Turner, G., and Bennett, J. W. (2005) Fungal secondary metabolism – from biochemistry to genomics, Nat. Rev. Microbiol., 3, 937-947, https://doi.org/10.1038/nrmicro1286.
Macheleidt, J., Mattern, D. J., Fischer, J., Netzker, T., Weber, J., Schroeckh, V., et al. (2016) Regulation and role of fungal secondary metabolites, Annu. Rev. Genet., 50, 371-392, https://doi.org/10.1146/annurev-genet-120215-035203.
Zarnowski, R., Sanchez, H., Covelli, A. S., Dominguez, E., Jaromin, A., Bernhardt, J., et al. (2018) Candida albicans biofilm-induced vesicles confer drug resistance through matrix biogenesis, PLoS Biol., 16, e2006872, https://doi.org/10.1371/journal.pbio.2006872.
Khandelwal, N. K., Wasi, M., Nair, R., Gupta, M., Kumar, M., Mondal, A. K., et al. (2019) Vacuolar sequestration of azoles, a novel strategy of azole antifungal resistance conserved across pathogenic and nonpathogenic yeast, Antimicrob. Agents Chemother., 63, e01347-18, https://doi.org/10.1128/AAC.01347-18.
Chang, W., Zhang, M., Zheng, S., Li, Y., Li, X., Li, W., et al. (2015) Trapping toxins within lipid droplets is a resistance mechanism in fungi, Sci. Rep., 5, 15133, https://doi.org/10.1038/srep15133.
Bush, K., and Bradford, P. A. (2019) Interplay between β-lactamases and new β-lactamase inhibitors, Nat. Rev. Microbiol., 17, 295-306, https://doi.org/10.1038/s41579-019-0159-8.
Hlavica, P. (2013) Evaluation of structural features in fungal cytochromes P450 predicted to rule catalytic diversification, Biochim. Biophys. Acta, 1834, 205-220, https://doi.org/10.1016/j.bbapap.2012.09.012.
Panwar, S. L., Pasrija, R., and Prasad, R. (2008) Membrane homoeostasis and multidrug resistance in yeast, Biosci. Rep., 28, 217-228, https://doi.org/10.1042/BSR20080071.
Cannon, R. D., Lamping, E., Holmes, A. R., Niimi, K., Baret, P. V., Keniya, M. V., et al. (2009) Efflux-mediated antifungal drug resistance, Clin. Microbiol. Rev., 22, 291-321, https://doi.org/10.1128/CMR.00051-08.
Gottesman, M. M., Fojo, T., and Bates, S. E. (2002) Multidrug resistance in cancer: role of ATP-dependent transporters, Nat. Rev. Cancer, 2, 48-58, https://doi.org/10.1038/nrc706.
Di, C., and Zhao, Y. (2015) Multiple drug resistance due to resistance to stem cells and stem cell treatment progress in cancer (review), Exp. Ther. Med., 9, 289-293, https://doi.org/10.3892/etm.2014.2141.
Lewis, K. (2020) The science of antibiotic discovery, Cell, 181, 29-45, https://doi.org/10.1016/j.cell.2020.02.056.
Fetisova, E. K., Avetisyan, A. V., Izyumov, D. S., Korotetskaya, M. V., Chernyak, B. V., and Skulachev, V. P. (2010) Mitochondria-targeted antioxidant SkQR1 selectively protects MDR (Pgp 170)-negative cells against oxidative stress, FEBS Lett., 584, 562-566, https://doi.org/10.1016/j.febslet.2009.12.002.
Nazarov, P. A., Osterman, I. A., Tokarchuk, A. V., Karakozova, M. V., Korshunova, G. A., et al. (2017) Mitochondria-targeted antioxidants as highly effective antibiotics, Sci. Rep., 7, 1394, https://doi.org/10.1038/s41598-017-00802-8.
Hlavácek, O., Kucerová, H., Harant, K., Palková, Z., and Váchová, L. (2009) Putative role for ABC multidrug exporters in yeast quorum sensing, FEBS Lett., 583, 1107-1113, https://doi.org/10.1016/j.febslet.2009.02.030.
Krasowska, A., Łukaszewicz, M., Bartosiewicz, D., and Sigler, K. (2010) Cell ATP level of Saccharomyces cerevisiae sensitively responds to culture growth and drug-inflicted variations in membrane integrity and PDR pump activity, Biochem. Biophys. Res. Commun., 395, 51-55, https://doi.org/10.1016/j.bbrc.2010.03.133.
Prunuske, A. J., Waltner, J. K., Kuhn, P., Gu, B., and Craig, E. A. (2012) Role for the molecular chaperones Zuo1 and Ssz1 in quorum sensing via activation of the transcription factor Pdr1, Proc. Natl. Acad. Sci. USA, 109, 472-477, https://doi.org/10.1073/pnas.1119184109.
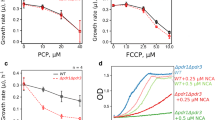
Galkina, K. V., Finkelberg, J. M., Markova, O. V., Azbarova, A. V., Banerjee, A., et al. (2020) Protonophore FCCP provides fitness advantage to PDR-deficient yeast cells, J. Bioenerg. Biomembr., 52, 383-395, https://doi.org/10.1007/s10863-020-09849-1.
Usher, J., and Haynes, K. (2019) Attenuating the emergence of anti-fungal drug resistance by harnessing synthetic lethal interactions in a model organism, PLoS Genet., 15, e1008259, https://doi.org/10.1371/journal.pgen.1008259.
Moye-Rowley, W. S. (2018) Multiple interfaces control activity of the Candida glabrata Pdr1 transcription factor mediating azole drug resistance, Curr. Genet., 65, 103–108, https://doi.org/10.1007/s00294-018-0870-4.
Boyer, J., Badis, G., Fairhead, C., Talla, E., Hantraye, F., et al. (2004) Large-scale exploration of growth inhibition caused by overexpression of genomic fragments in Saccharomyces cerevisiae, Genome Biol., 5, 72, https://doi.org/10.1186/gb-2004-5-9-r72.
Wale, N., Sim, D. G., Jones, M. J., Salathe, R., Day, T., and Read, A. F. (2017) Resource limitation prevents the emergence of drug resistance by intensifying within-host competition, Proc. Natl. Acad. Sci. USA, 114, 13774-13779, https://doi.org/10.1073/pnas.1715874115.
Prasad, R., and Goffeau, A. (2012) Yeast ATP-binding cassette transporters conferring multidrug resistance, Annu. Rev. Microbiol., 66, 39-63, https://doi.org/10.1146/annurev-micro-092611-150111.
Moreno, A., Banerjee, A., Prasad, R., and Falson, P. (2019) PDR-like ABC systems in pathogenic fungi, Res. Microbiol., 170, 417-425, https://doi.org/10.1016/j.resmic.2019.09.002.
Tsao, S., Rahkhoodaee, F., and Raymond, M. (2009) Relative contributions of the Candida albicans ABC transporters Cdr1p and Cdr2p to clinical azole resistance, Antimicrob. Agents Chemother., 53, 1344-1352, https://doi.org/10.1128/AAC.00926-08.
Wasi, M., Khandelwal, N. K., Moorhouse, A. J., Nair, R., Vishwakarma, P., Bravo Ruiz, G., et al. (2019) ABC Transporter genes show upregulated expression in drug-resistant clinical isolates of Candida auris: a genome-wide characterization of ATP-binding cassette (ABC) transporter genes, Front. Microbiol., 10, 1445, https://doi.org/10.3389/fmicb.2019.01445.
Cowen, L. E., and Lindquist, S. (2005) Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi, Science, 309, 2185-2189, https://doi.org/10.1126/science.1118370.
Sauna, Z. E., Bohn, S. S., Rutledge, R., Dougherty, M. P., Cronin, S., May, L., et al. (2008) Mutations define cross-talk between the N-terminal nucleotide-binding domain and transmembrane helix-2 of the yeast multidrug transporter Pdr5: possible conservation of a signaling interface for coupling ATP hydrolysis to drug transport, J. Biol. Chem., 283, 35010-35022, https://doi.org/10.1074/jbc.M806446200.
Kim, Y., and Chen, J. (2018) Molecular structure of human P-glycoprotein in the ATP-bound, outward-facing conformation, Science, 359, 915-919, https://doi.org/10.1126/science.aar7389.
Ernst, R., Kueppers, P., Stindt, J., Kuchler, K., and Schmitt, L. (2010) Multidrug efflux pumps: substrate selection in ATP-binding cassette multidrug efflux pumps – first come, first served? FEBS J., 277, 540-549, https://doi.org/10.1111/j.1742-4658.2009.07485.x.
Gupta, R. P., Kueppers, P., Schmitt, L., and Ernst, R. (2011) The multidrug transporter Pdr5: a molecular diode? Biol. Chem., 392, 53-60, https://doi.org/10.1515/BC.2011.011.
Hofmann, S., Januliene, D., Mehdipour, A. R., Thomas, C., Stefan, E., Brüchert, S., et al. (2019) Conformation space of a heterodimeric ABC exporter under turnover conditions, Nature, 571, 580-583, https://doi.org/10.1038/s41586-019-1391-0.
Ernst, R., Kueppers, P., Klein, C. M., Schwarzmueller, T., Kuchler, K., and Schmitt, L. (2008) A mutation of the H-loop selectively affects rhodamine transport by the yeast multidrug ABC transporter Pdr5, Proc. Natl. Acad. Sci. USA, 105, 5069-5074, https://doi.org/10.1073/pnas.0800191105.
Decottignies, A., Kolaczkowski, M., Balzi, E., and Goffeau, A. (1994) Solubilization and characterization of the overexpressed PDR5 multidrug resistance nucleotide triphosphatase of yeast, J. Biol. Chem., 269, 12797-12803.
Downes, M. T., Mehla, J., Ananthaswamy, N., Wakschlag, A., Lamonde, M., et al. (2013) The transmission interface of the Saccharomyces cerevisiae multidrug transporter Pdr5: Val-656 located in intracellular loop 2 plays a major role in drug resistance, Antimicrob. Agents Chemother., 57, 1025-034, https://doi.org/10.1128/AAC.02133-12.
Golin, J., Kon, Z. N., Wu, C.-P., Martello, J., Hanson, L., et al. (2007) Complete inhibition of the Pdr5p multidrug efflux pump ATPase activity by its transport substrate clotrimazole suggests that GTP as well as ATP may be used as an energy source, Biochemistry, 46, 13109-13119, https://doi.org/10.1021/bi701414f.
Wagner, M., Smits, S. H. J., and Schmitt, L. (2019) In vitro NTPase activity of highly purified Pdr5, a major yeast ABC multidrug transporter, Sci. Rep., 9, 7761, https://doi.org/10.1038/s41598-019-44327-8.
Golin, J., and Ambudkar, S. V. (2015) The multidrug transporter Pdr5 on the 25th anniversary of its discovery: an important model for the study of asymmetric ABC transporters, Biochem. J., 467, 353-363, https://doi.org/10.1042/BJ20150042.
Ho, B., Baryshnikova, A., and Brown, G. W. (2018) Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome, Cell Syst., 6, 192-205, https://doi.org/10.1016/j.cels.2017.12.004.
Sheldon, J. G., Williams, S. P., Fulton, A. M., and Brindle, K. M. (1996) 31P NMR magnetization transfer study of the control of ATP turnover in Saccharomyces cerevisiae, Proc. Natl. Acad. Sci. USA, 93, 6399-6404, https://doi.org/10.1073/pnas.93.13.639.9.
Ramirez-Gaona, M., Marcu, A., Pon, A., Guo, A. C., Sajed, T., et al. (2017) YMDB 2.0: a significantly expanded version of the yeast metabolome database, Nucleic Acids Res., 45, 440-445, https://doi.org/10.1093/nar/gkw1058.
Kolaczkowski, M., van der Rest, M., Cybularz-Kolaczkowska, A., Soumillion, J. P., Konings, W. N., and Goffeau, A. (1996) Anticancer drugs, ionophoric peptides, and steroids as substrates of the yeast multidrug transporter Pdr5p, J. Biol. Chem., 271, 31543-31548, https://doi.org/10.1074/jbc.271.49.31543.
Wilcox, L. J., Balderes, D. A., Wharton, B., Tinkelenberg, A. H., Rao, G., and Sturley, S. L. (2002) Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast, J. Biol. Chem., 277, 32466-32472, https://doi.org/10.1074/jbc.M204707200.
Zhu, J., Krom, B. P., Sanglard, D., Intapa, C., Dawson, C. C., Peters, B. M., et al. (2011) Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione, PLoS One, 6, e28830, https://doi.org/10.1371/journal.pone.0028830.
Cabrito, T. R., Teixeira, M. C., Singh, A., Prasad, R., and Sá-Correia, I. (2011) The yeast ABC transporter Pdr18 (ORF YNR070w) controls plasma membrane sterol composition, playing a role in multidrug resistance, Biochem. J., 440, 195-202, https://doi.org/10.1042/BJ20110876.
Godinho, C. P., Dias, P. J., Ponçot, E., and Sá-Correia, I. (2018) The paralogous genes PDR18 and SNQ2, encoding multidrug resistance ABC transporters, derive from a recent duplication event, PDR18 being specific to the Saccharomyces genus, Front. Genet., 9, 476, https://doi.org/10.3389/fgene.2018.00476.
Salin, H., Fardeau, V., Piccini, E., Lelandais, G., Tanty, V., et al. (2008) Structure and properties of transcriptional networks driving selenite stress response in yeasts, BMC Genomics, 9, 333, https://doi.org/10.1186/1471-2164-9-333.
Shahi, P., and Moye-Rowley, W. S. (2009) Coordinate control of lipid composition and drug transport activities is required for normal multidrug resistance in fungi, Biochim. Biophys. Acta, 1794, 852-859, https://doi.org/10.1016/j.bbapap.2008.12.012.
Montañés, F. M., Pascual-Ahuir, A., and Proft, M. (2011) Repression of ergosterol biosynthesis is essential for stress resistance and is mediated by the Hog1 MAP kinase and the Mot3 and Rox1 transcription factors, Mol. Microbiol., 79, 1008-1023, https://doi.org/10.1111/j.1365-2958.2010.07502.x.
Sokolov, S. S., Trushina, N. I., Severin, F. F., and Knorre, D. A. (2019) Ergosterol turnover in yeast: an interplay between biosynthesis and transport, Biochemistry, 84, 346-357, https://doi.org/10.1134/S0006297919040023.
Godinho, C. P., Prata, C. S., Pinto, S. N., Cardoso, C., Bandarra, N. M., et al. (2018) Pdr18 is involved in yeast response to acetic acid stress counteracting the decrease of plasma membrane ergosterol content and order, Sci. Rep., 8, 7860, https://doi.org/10.1038/s41598-018-26128-7.
Kodedová, M., and Sychrová, H. (2015) Changes in the sterol composition of the plasma membrane affect membrane potential, salt tolerance and the activity of multidrug resistance pumps in Saccharomyces cerevisiae, PLoS One, 10, e0139306, https://doi.org/10.1371/journal.pone.0139306.
Liu, S.-L., Sheng, R., Jung, J. H., Wang, L., Stec, E., et al. (2017) Orthogonal lipid sensors identify transbilayer asymmetry of plasma membrane cholesterol, Nat. Chem. Biol., 13, 268-274, https://doi.org/10.1038/nchembio.2268.
Solanko, L. M., Sullivan, D. P., Sere, Y. Y., Szomek, M., Lunding, A., et al. (2018) Ergosterol is mainly located in the cytoplasmic leaflet of the yeast plasma membrane, Traffic, 19, 198-214, https://doi.org/10.1111/tra.12545.
Garrigues, A., Escargueil, A. E., and Orlowski, S. (2002) The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane, Proc. Natl. Acad. Sci. USA, 99, 1034710352, https://doi.org/10.1073/pnas.162366399.
Pomorski, T., Holthuis, J. C. M., Herrmann, A., and van Meer, G. (2004) Tracking down lipid flippases and their biological functions, J. Cell. Sci., 117, 805-813, https://doi.org/10.1242/jcs.01055.
Kean, L. S., Grant, A. M., Angeletti, C., Mahé, Y., Kuchler, K., et al. (1997) Plasma membrane translocation of fluorescent-labeled phosphatidylethanolamine is controlled by transcription regulators, PDR1 and PDR3, J. Cell. Biol., 138, 255-270, https://doi.org/10.1083/jcb.138.2.255.
Decottignies, A., Grant, A. M., Nichols, J. W., de Wet, H., McIntosh, D. B., and Goffeau, A. (1998) ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p, J. Biol. Chem., 273, 12612-2622, https://doi.org/10.1074/jbc.273.20.12612.
Kihara, A., and Igarashi, Y. (2004) Cross talk between sphingolipids and glycerophospholipids in the establishment of plasma membrane asymmetry, Mol. Biol. Cell, 15, 4949-4959, https://doi.org/10.1091/mbc.e04-06-0458.
Hornby, J. M., Jensen, E. C., Lisec, A. D., Tasto, J. J., Jahnke, B., et al. (2001) Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol, Appl. Environ. Microbiol., 67, 2982-2992, https://doi.org/10.1128/AEM.67.7.2982-2992.2001.
Chen, H., and Fink, G. R. (2006) Feedback control of morphogenesis in fungi by aromatic alcohols, Genes Dev., 20, 1150-1161, https://doi.org/10.1101/gad.1411806.
Wongsuk, T., Pumeesat, P., and Luplertlop, N. (2016) Fungal quorum sensing molecules: role in fungal morphogenesis and pathogenicity, J. Basic Microbiol., 56, 440-447, https://doi.org/10.1002/jobm.201500759.
Enjalbert, B., and Whiteway, M. (2005) Release from quorum-sensing molecules triggers hyphal formation during Candida albicans resumption of growth, Eukaryot. Cell, 4, 1203-1210, https://doi.org/10.1128/EC.4.7.1203-1210.2005.
Davis-Hanna, A., Piispanen, A. E., Stateva, L. I., and Hogan, D. A. (2007) Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis Internet, Mol. Microbiol., 67, 47-62, https://doi.org/10.1111/j.1365-2958.2007.06013.x.
Sharma, M., and Prasad, R. (2011) The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans, Antimicrob. Agents Chemother., 55, 4834-4843, https://doi.org/10.1128/AAC.00344-11.
Jakab, Á., Tóth, Z., Nagy, F., Nemes, D., Bácskay, I., et al. (2019) Physiological and transcriptional responses of Candida parapsilosis to exogenous tyrosol, Appl. Environ. Microbiol., 85, https://doi.org/10.1128/AEM.01388-19.
Teixeira, M. C., and Sá-Correia, I. (2002) Saccharomyces cerevisiae resistance to chlorinated phenoxyacetic acid herbicides involves Pdr1p-mediated transcriptional activation of TPO1 and PDR5 genes, Biochem. Biophys. Res. Commun., 292, 530-537, https://doi.org/10.1006/bbrc.2002.6691.
Thakur, J. K., Arthanari, H., Yang, F., Pan, S.-J., Fan, X., Breger, J., et al. (2008) A nuclear receptor-like pathway regulating multidrug resistance in fungi, Nature, 452, 604-609, https://doi.org/10.1038/nature06836.
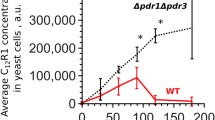
Galkina, K. V., Besedina, E. G., Zinovkin, R. A., Severin, F. F., and Knorre, D. A. (2018) Penetrating cations induce pleiotropic drug resistance in yeast, Sci. Rep., 8, 8131, https://doi.org/10.1038/s41598-018-26435-z.
Hallstrom, T. C., and Moye-Rowley, W. S. (2000) Multiple signals from dysfunctional mitochondria activate the pleiotropic drug resistance pathway in Saccharomyces cerevisiae, J. Biol. Chem., 275, 37347-37356, https://doi.org/10.1074/jbc.M007338200.
Zhang, X., and Moye-Rowley, W. S. (2001) Saccharomyces cerevisiae multidrug resistance gene expression inversely correlates with the status of the F(0) component of the mitochondrial ATPase, J. Biol. Chem., 276, 47844-47852, https://doi.org/10.1074/jbc.M106285200.
Kotiadis, V. N., Leadsham, J. E., Bastow, E. L., Gheeraert, A., Whybrew, J. M., et al. (2012) Identification of new surfaces of cofilin that link mitochondrial function to the control of multi-drug resistance, J. Cell. Sci., 125, 2288-2299, https://doi.org/10.1242/jcs.099390.
Chen, K.-H., Miyazaki, T., Tsai, H.-F., and Bennett, J. E. (2007) The bZip transcription factor Cgap1p is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata, Gene, 386, 63-72, https://doi.org/10.1016/j.gene.2006.08.010.
Galkina, K. V., Okamoto, M., Chibana, H., Knorre, D. A., and Kajiwara, S. (2019) Deletion of CDR1 reveals redox regulation of pleiotropic drug resistance in Candida glabrata, Biochimie, 170, 49-56, https://doi.org/10.1016/j.biochi.2019.12.002.
Belenky, P., Camacho, D., and Collins, J. J. (2013) Fungicidal drugs induce a common oxidative-damage cellular death pathway, Cell Rep., 3, 350-358, https://doi.org/10.1016/j.celrep.2012.12.021.
Mamnun, Y. M., Schüller, C., and Kuchler, K. (2004) Expression regulation of the yeast PDR5 ATP-binding cassette (ABC) transporter suggests a role in cellular detoxification during the exponential growth phase, FEBS Lett., 559, 111-117, https://doi.org/10.1016/S0014-5793(04)00046-8.
Cadek, R., Chládková, K., Sigler, K., and Gásková, D. (2004) Impact of the growth phase on the activity of multidrug resistance pumps and membrane potential of S. cerevisiae: effect of pump overproduction and carbon source, Biochim. Biophys. Acta, 1665, 111-117, https://doi.org/10.1016/j.bbamem.2004.06.020.
Rahman, H., Carneglia, J., Lausten, M., Robertello, M., Choy, J., and Golin, J. (2018) Robust, pleiotropic drug resistance 5 (Pdr5)-mediated multidrug resistance is vigorously maintained in Saccharomyces cerevisiae cells during glucose and nitrogen limitation, FEMS Yeast Res., 18, https://doi.org/10.1093/femsyr/foy029.
Vu, B. G., Thomas, G. H., and Moye-Rowley, W. S. (2019) Evidence that ergosterol biosynthesis modulates activity of the Pdr1 transcription factor in Candida glabrata, MBio, 10, https://doi.org/10.1128/mBio.00934-19.
Sokolov, S. S., Vorobeva, M. A., Smirnova, A. I., Smirnova, E. A., Trushina, N. I., et al. (2020) LAM Genes contribute to environmental stress tolerance but sensibilize yeast cells to azoles, Front. Microbiol., 11, 38, https://doi.org/10.3389/fmicb.2020.00038.
Azbarova, A. V., Galkina, K. V., Sorokin, M. I., Severin, F. F., and Knorre, D. A. (2017) The contribution of Saccharomyces cerevisiae replicative age to the variations in the levels of Trx2p, Pdr5p, Can1p and Idh isoforms, Sci. Rep., 7, 13220, https://doi.org/10.1038/s41598-017-13576-w.
Liu, J., François, J.-M., and Capp, J.-P. (2016) Use of noise in gene expression as an experimental parameter to test phenotypic effects, Yeast, 33, 209-216, https://doi.org/10.1002/yea.3152.
Knorre, D. A., Azbarova, A. V., Galkina, K. V., Feniouk, B. A., and Severin, F. F. (2018) Replicative aging as a source of cell heterogeneity in budding yeast, Mech. Ageing Dev., 176, 24-31, https://doi.org/10.1016/j.mad.2018.09.001.
Bódi, Z., Farkas, Z., Nevozhay, D., Kalapis, D., Lázár, V., et al. (2017) Phenotypic heterogeneity promotes adaptive evolution, PLoS Biol., 15, e2000644, https://doi.org/10.1371/journal.pbio.2000644.
Acknowledgements
We are grateful to V. P. Skulachev for stimulating discussions on basic principles of multiple drug resistance during the seminars on bioenergetics organized by him.
Funding
DK and RP acknowledge support from the joint Indo-Russian grant INT/RUS/RFBR/P-328 (India)/Russian Foundation for Basic Research [grant 18-54-45001 IND-A (Russia)]. The study was also supported by the Russian Science Foundation (grant 20-14-00268, for DK and KG, the chapter on the bioenergetic cost of PDR-transporters). This work was also supported by Moscow State University Grant for Leading Scientific Schools “Depository of the Living Systems” in the framework of the MSU Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Knorre, D.A., Galkina, K.V., Shirokovskikh, T. et al. Do Multiple Drug Resistance Transporters Interfere with Cell Functioning under Normal Conditions?. Biochemistry Moscow 85, 1560–1569 (2020). https://doi.org/10.1134/S0006297920120081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920120081