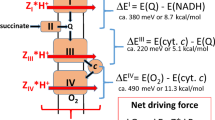
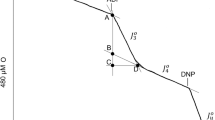
Abstract
The mechanism of oxidative phosphorylation and its regulation remain one of the main problems of bioenergetics. Efficiency of the mitochondrial energization is determined by the relationship between the rate of generation of electrochemical potential of hydrogen ions and the rate of its expenditure on the synthesis of ATP and the use of ATP in endergonic reactions. Uncoupling (partial or complete), which occurs in the process of uncontrolled and controlled leakage of ions through the inner mitochondrial membrane, on the one hand leads to the decrease in the relative synthesis of ATP, and on the other, being consistent with the law of conservation of energy, leads to the formation of heat, generation of which is an essential function of the organism. In addition to increased thermogenesis, the increase of non-phosphorylating oxidation of various substrates is accompanied by the decrease in transmembrane potential, production of reactive oxygen species, and activation of oxygen consumption, water and carbon dioxide production, increase in the level of intracellular ADP and acidification of the cytosol. In this analysis, each of these factors will be considered separately for its role in regulating metabolism.


Similar content being viewed by others
References
Brand, M. D., Chien, L. F., Ainscow, E. K., Rolfe, D. F., and Porter, R. K. (1994) The causes and functions of mitochondrial proton leak, Biochim. Biophys. Acta, 1187, 132-139, https://doi.org/10.1016/0005-2728(94)90099-x.
Skulachev, V. P., and Maslov, S. P. (1960) The role of oxidation in thermopregulation, Biochemistry (Moscow), 25, 1058-1064.
Skulachev, V. P. (1961) Regulation of the coupling of oxidation and phosphorylation, 5th Internat. Cong. of Biochem. (Moscow), 5, 367-373.
Skulachev, V. P., Maslov, S. P., Sivkova, V. G., Kalinichenko, L. P., and Maslova, G. M. (1963) Cold-induced uncoupling of oxidation and phosphorylation in muscles of white mice, Biochemistry (Moscow), 28, 70-79.
Nedergaard, J., and Cannon, B. (2018) Brown adipose tissue as a heat-producing thermoeffector, Handb. Clin. Neurol., 156, 137-152, https://doi.org/10.1016/B978-0-444-63912-7.00009-6.
Yaniv, Y., Juhaszova, M., Nuss, H. B., Wang, S., Zorov, D. B., Lakatta, E. G., and Sollott, S. J. (2010) Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives, Ann. N. Y. Acad. Sci., 1188, 133-142, https://doi.org/10.1111/j.1749-6632.2009.05093.x.
Maley, G. F., and Lardi, H. A. (1953) Metabolic effects of thyroid hormones in vitro. II. Influence of thyroxine and triiodothyronine on oxidative phosphorylation, J. Biol. Chem., 204, 435-444, PMID: 13084614.
Hoch, F. L., and Lipmann, F. (1954) The uncoupling of respiration and phosphorylation by thyroid hormones, Proc. Natl. Acad. Sci. USA, 40, 909-921, https://doi.org/10.1073/pnas.40.10.909.
Dickens, F., and Salmony, D. (1956) Effects of thyroid hormones in vitro on tissue respiration, oxidative phosphorylation and the swelling of mitochondria, Biochem. J., 64, 645-651, https://doi.org/10.1042/bj0640645.
Skulachev, V. P. (1998) Uncoupling: new approaches to an old problem of bioenergetics, Biochim. Biophys. Acta, 1363, 100-124, https://doi.org/10.1016/s0005-2728(97)00091-1.
Korshunov, S. S., Skulachev, V. P., and Starkov, A. A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria, FEBS Lett., 416, 15-18, https://doi.org/10.1016/s0014-5793(97)01159-9.
Zorov, D. B., Krasnikov, B. F., Kuzminova, A. E., Vysokikh, M. Yu., and Zorova, L. D. (1997) Mitochondria revisited. Alternative functions of mitochondria, Bios. Rep., 17, 507-520, https://doi.org/10.1023/a:1027304122259.
Zorov, D. B., Filburn, C. R., Klotz, L. O., Zweier, J. L., and Sollott, S. J. (2000) Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes, J. Exp. Med., 192, 1001-1014, https://doi.org/10.1084/jem.192.7.1001.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2006) Mitochondrial ROS-induced ROS release: an update and review, Biochim. Biophys. Acta, 1757, 509-517, https://doi.org/10.1016/j.bbabio.2006.04.029.
Zorov, D. B., Juhaszova, M., and Sollott, S. J. (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release, Physiol. Rev., 94, 909-950, https://doi.org/10.1152/physrev.00026.2013.
Zorov, D. B., Bannikova, S. Y., Belousov, V. V., Vyssokikh, M. Y., Zorova, L. D., Isaev, N. K., Krasnikov, B. F., and Plotnikov, E. Y. (2005) Reactive oxygen and nitrogen species: friends or foes, Biochemistry (Moscow), 70, 215-221.
Liberman, E. A., Topaly, V. P., Tsofina, L. M., Jasaitis, A. A., and Skulachev, V. P. (1969) Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria, Nature, 222, 1076-1078, https://doi.org/10.1038/2221076a0.
Andreyev, A. Yu., Bondareva, T. O., Dedukhova, V. I., Mokhova, E. N., Skulachev, V. P., Tsofina, L. M., Volkov, N. I., and Vygodina, T. V. (1989) The ATP/ADP-antiporter is involved in the uncoupling effect of fatty acids on mitochondria, Eur. J. Biochem., 182, 585-592, https://doi.org/10.1111/j.1432-1033.1989.tb14867.x.
Wieckowski, M. R., and Wojtczak, L. (1997) Involvement of the dicarboxylate carrier in the protonophoric action of long-chain fatty acids in mitochondria, Biochem. Biophys. Res. Commun., 232, 414-417, https://doi.org/10.1006/bbrc.1997.6298.
Samartsev, V. N., Smirnov, A. V., Zeldi, I. P., Markova, O. V., Mokhova, E. N., and Skulachev, V. P. (1997) Involvement of aspartate/glutamate antiporter in fatty acid-induced uncoupling of liver mitochondria, Biochim. Biophys. Acta, 1319, 251-257, https://doi.org/10.1016/s0005-2728(96)00166-1.
Rupprecht, A., Sokolenko, E. A., Beck, V., Ninnemann, O., Jaburek, M., Trimbuch, T., Klishin, S. S., Jezek, P., Skulachev, V. P., and Pohl, E. E. (2010) Role of the transmembrane potential in the membrane protein leak, Biophys. J., 98, 1503-1511, https://doi.org/10.1016/j.bpj.2009.12.4301.
Bertholet, A. M., Chouchani, E. T., Kazak, L., Angelin, A., Fedorenko, A., et al. (2019) H+ transport is an integral function of the mitochondrial ADP/ATP carrier, Nature, 571, 515-520, https://doi.org/10.1038/s41586-019-1400-3.
Chrétien, D., Bénit, P., Ha, H. H., Keipert, S., El-Khoury, R., et al. (2018) Mitochondria are physiologically maintained at close to 50°C, PLoS Biol., 16, e2003992, https://doi.org/10.1371/journal.pbio.2003992.
Starkov, A. A., Dedukhova, V. I., and Skulachev, V. P. (1994) 6-ketocholestanol abolishes the effect of the most potent uncouplers of oxidative phosphorylation in mitochondria, FEBS Lett., 355, 305-308, https://doi.org/10.1016/0014-5793(94)01211-3.
Lehninger, A. L. (1962) Water uptake and extrusion by mitochondria in relation to oxidative phosphorylation, Physiol. Rev., 42, 467-517, https://doi.org/10.1152/physrev.1962.42.3.467.
Solenski, N. J., diPierro, C. G., Trimmer, P. A., Kwan, A. L., and Helm, G. A. (2002) Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia, Stroke, 33, 816-824, https://doi.org/10.1161/hs0302.104541.
Kaasik, A., Kuum, M., Joubert, F., Wilding, J., Ventura-Clapier, R., and Veksler, V. (2010) Mitochondria as a source of mechanical signals in cardiomyocytes, Cardiovasc. Res., 87, 83-91, https://doi.org/10.1093/cvr/cvq039.
Zorov, D. B., Vorobjev, I. A., Popkov, V. A., Babenko, V. A., Zorova, L. D., et al. (2019) Lessons from the discovery of mitochondrial fragmentation (fission): a review and update, Cells, 8, 175, https://doi.org/10.3390/cells8020175.
Ware, A. J., D’Agostino, A. N., and Combes, B. (1971) Cerebral edema: a major complication of massive hepatic necrosis, Gastroenterology, 61, 877-884, PMID: 125688.
Durward, Q. J., Del Maestro, R. F., Amacher, A. L., and Farrar, J. K. (1983) The influence of systemic arterial pressure and intracranial pressure on the development of cerebral vasogenic edema, J. Neurosurg., 59, 803-809, https://doi.org/10.3171/jns.1983.59.5.0803.
Preston, E., and Webster, J. (2004) A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood-brain barrier after ischemia, Acta Neuropathol., 108, 406-412, https://doi.org/10.1007/s00401-004-0905-4.
Schmidt, B., McCracken, J., and Ferguson-Miller, S. (2003) A discrete water exit pathway in the membrane protein cytochrome c oxidase, Proc. Natl. Acad. Sci. USA, 100, 15539-15542, https://doi.org/10.1073/pnas.2633243100.
Zimmerberg, J., and Parsegian, V. A. (1987) Water movement during channel opening and closing, J. Bioenerg. Biomembr., 19, 351-358, https://doi.org/10.1007/BF00768538.
Gena, P., Fanelli, E., Brenner, C., Svelto, M., and Calamita, G. (2009) News and views on mitochondrial water transport, Front. Biosci. (Landmark Ed.), 14, 4189-4419, https://doi.org/10.2741/3522.
Lee, W. K., and Thévenod, F. (2006) A role for mitochondrial aquaporins in cellular life-and-death decisions? Am. J. Physiol. Cell Physiol., 291, 195-202, https://doi.org/10.1152/ajpcell.00641.2005.
Calamita, G., Gena, P., Meleleo, D., Ferri, D., and Svelto, M. (2006) Water permeability of rat liver mitochondria: a biophysical study, Biochim. Biophys. Acta, 1758, 1018-1024, https://doi.org/10.1016/j.bbamem.2006.07.008.
Saparov, S. M., Liu, K., Agre, P., and Pohl, P. (2007) In vitro analysis and modification of aquaporin pore selectivity, J. Biol. Chem., 282, 5296-5301, https://doi.org/10.1074/jbc.M609343200.
Juhaszova, M., Zorov, D. B., Kim, S. H., Pepe, S., Fu, Q., et al. (2004) Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore, J. Clin. Invest., 113, 1535-1549, https://doi.org/10.1172/JCI19906.
Halestrap, A. P. (1989) The regulation of the matrix volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism, Biochim. Biophys. Acta, 973, 355-382, https://doi.org/10.1016/s0005-2728(89)80378-0.
Konstantinova, S. A., Mannella, C. A., Skulachev, V. P., and Zorov, D. B. (1995) Immunoelectron microscopic study of the distribution of porin on outer membranes of rat heart mitochondria, J. Bioenerg. Biomembr., 27, 93-99, https://doi.org/10.1007/BF02110336.
Maeder, C. I., Shen, K., and Hoogenraad, C. C., (2014) Axon and dendritic trafficking, Curr. Opin. Neurobiol., 27, 165-170, https://doi.org/10.1016/j.conb.2014.03.015.
Melkov, A., and Abdu, U. (2018) Regulation of long-distance transport of mitochondria along microtubules, Cell. Mol. Life Sci., 75, 163-176, https://doi.org/10.1007/s00018-017-2590-1.
Zorova, L. D., Popkov, V. A., Plotnikov, E. Y., Silachev, D. N., Pevzner, I. B., et al. (2018) Mitochondrial membrane potential, Anal. Biochem., 552, 50-59, https://doi.org/10.1016/j.ab.2017.07.009.
Rizack, M. A. (1964) An epinephrine-sensitive lipolytic activity in adipose tissue, J. Biol. Chem., 236, 657-662, PMID: 14169136.
Eastman, A. (1994) Deoxyribonuclease II in apoptosis and the significance of intracellular acidification, Cell Death Differ., 1, 7-9, PMID: 17180001.
Gottlieb, R. A., Giesing, H. A., Zhu, J. Y., Engler, R. L., and Babior, B. M. (1995) Cell acidification in apoptosis: granulocyte colony-stimulating factor delays programmed cell death in neutrophils by up-regulating the vacuolar H(+)-ATPase, Proc. Natl. Acad. Sci. USA, 92, 5965-5968, https://doi.org/10.1073/pnas.92.13.5965.
Silachev, D. N., Gulyaev, M. V., Zorova, L. D., Khailova, L. S., Gubsky, L. V., et al. (2015) Magnetic resonance spectroscopy of the ischemic brain under lithium treatment. Link to mitochondrial disorders under stroke, Chem. Biol. Interact., 237, 175-182, https://doi.org/10.1016/j.cbi.2015.06.012.
Steinberg, G. R., and Kemp, B. E. (2009) AMPK in health and disease, Physiol. Rev., 89, 1025-1078, https://doi.org/10.1152/physrev.00011.2008.
Zhang, Q., Raoof, M., Chen, Y., Sumi, Y., Sursal, T., Junger, W., Brohi, K., Itagaki, K., and Hauser, C. J. (2010) Circulating mitochondrial DAMPs cause inflammatory responses tom injury, Nature, 464, 104-107, https://doi.org/10.1038/nature08780.
Borea, P. A., Gessi, S., Merighi, S., Vincenzi, F., and Varani, K. (2015) Pharmacology of adenosine receptors: the state of the art, Physiol. Rev., 98, 1591-1625, https://doi.org/10.1152/physrev.00049.2017.
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., and Nedergaard, M. (2008) Connexin 43 hemichannels are permeable to ATP, J. Neurosci., 28, 4702-4711, https://doi.org/10.1523/JNEUROSCI.5048-07.2008.
Chekeni, F. B., Elliott, M. R., Sandilos, J. K., Walk, S. F., Kinchen, J. M., et al. (2010) Pannexin 1 channels mediate “find-me” signal release and membrane permeability during apoptosis, Nature, 467, 863-867, https://doi.org/10.1038/nature09413.
Roos, A., and Boron, W. F. (1981) Intracellular pH, Physiol. Rev., 61, 296-434, https://doi.org/10.1152/physrev.1981.61.2.296.
Aickin, C. C. (1986) Intracellular pH regulation by vertebrate muscle, Annu. Rev. Physiol., 48, 349-361, https://doi.org/10.1146/annurev.ph.48.030186.002025.
Inserte, J., Barba, I., Hernando, V., Abellán, A., Ruiz-Meana, M., Rodríguez-Sinovas, A., and Garcia-Dorado, D. (2008) Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage, Cardiovasc. Res., 77, 782-790, https://doi.org/10.1093/cvr/cvm082.
Pullman, M. E., and Monroe, G. C. (1963) A naturally occurring inhibitor of mitochondrial adenosine triphosphatase, J. Biol. Chem., 238, 3762-3769, PMID: 14109217.
Di Lisa, F., Blank, P. S., Colonna, R., Gambassi, G., Silverman, H. S., Stern, M. D., and Hansford, R. G. (1995) Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition, J. Physiol., 486, 1-13, https://doi.org/10.1113/jphysiol.1995.sp020786.
Queliconi, B. B., Kowaltowski, A. J., and Gottlieb, R. A. (2016) Bicarbonate increases ischemia-reperfusion damage by inhibiting mitophagy, PLoS One, 11, e0167678, https://doi.org/10.1371/journal.pone.0167678.
Zorov, D. B., Popkov, V. A., Zorova, L. D., Vorobjev, I. A., Pevzner, I. B., et al. (2017) Mitochondrial aging: is there a mitochondrial clock? J. Gerontol. A Biol. Sci. Med. Sci., 72, 1171-1179, https://doi.org/10.1093/gerona/glw184.
Liochev, S. I., and Fridovich, I. (2002) Copper, zinc superoxide dismutase and H2O2. Effects of bicarbonate on inactivation and oxidations of NADPH and urate, and on consumption of H2O2, J. Biol. Chem., 277, 34674-34678, https://doi.org/10.1074/jbc.M204726200.
Medinas, D. B., Cerchiaro, G., Trindade, D. F., and Augusto, O. (2007) The carbonate radical and related oxidants derived from bicarbonate buffer, IUBMB Life, 59, 255-262, https://doi.org/10.1080/15216540701230511.
Queiroz, R. F., Paviani, V., Coelho, F. R., Marques, E. F., Di Mascio, P., and Augusto, O. (2013) The carbonylation and covalent dimerization of human superoxide dismutase 1 caused by its bicarbonate-dependent peroxidase activity is inhibited by the radical scavenger tempol, Biochem. J., 455, 37-46, https://doi.org/10.1042/BJ20130180.
Queliconi, B. B., Marazzi, T. B., Vaz, S. M., Brookes, P. S., Nehrke, K., Augusto, O., and Kowaltowski, A. J. (2013) Bicarbonate modulates oxidative and functional damage in ischemia-reperfusion, Free Radic. Biol. Med., 55, 46-53, https://doi.org/10.1016/j.freeradbiomed.2012.11.007.
Kumar, S., Flacke, J., Kostin, S., Appukuttan, A., Reusch, H. P., and Ladilov, Y. (2011) SLC4A7 sodium bicarbonate cotransporter controls mitochondrial apoptosis in ischaemic coronary endothelial cells, Cardiovasc. Res., 89, 392-400, https://doi.org/10.1093/cvr/cvq330.
Alka, K., and Casey, J. R. (2014) Bicarbonate transport in health and disease, IUBMB Life, 66, 596-615, https://doi.org/10.1002/iub.1315.
Nozik-Grayck, E., Huang, Y.-C. T., Carraway, M. S., and Piantadosi, C. (2003) Bicarbonate-dependent superoxide release and pulmonary artery tone, Am. J. Physiol. Heart Circ. Physiol., 285, 2327-2335, https://doi.org/10.1152/ajpheart.00507.2003.
Acin-Perez, R., Salazar, E., Kamenetsky, M., Buck, J., Levin, L. R., and Manfredi, G. (2009) Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation, Cell Metabol., 9, 265-276, https://doi.org/10.1016/j.cmet.2009.01.012.
Zeylemaker, W. P., Klaasse, A. D., Slater, E. C., and Veeger, C. (1970) Studies on succinate dehydrogenase. VI. Inhibition by monocarboxylic acids, Biochim. Biophys. Acta, 198, 415-422, https://doi.org/10.1016/0005-2744(70)90120-8.
Kasho, V. N., and Boyer, P. D. (1984) Relationships of inosine triphosphate and bicarbonate effects on F1-ATPase to the binding change mechanism, J. Bioenerg. Biomembr., 16, 407-419, https://doi.org/10.1007/BF00743235.
Roveri, O. A., and Calcaterra, N. B. (1985) Steady-state kinetic of F1-ATPase. Mechanism of anion activation, FEBS Lett., 192, 123-127, https://doi.org/10.1016/0014-5793(85)80056-9.
Lodeyro, A. F., Calcaterra, N. B., and Roveri, O. A. (2001) Inhibition of steady-state mitochondrial ATP synthesis by bicarbonate, an activating anion of ATP hydrolysis, Biochim. Biophys. Acta, 1506, 236-243, https://doi.org/10.1016/s0005-2728(01)00221-3.
Khailova, L. S., Vygodina, T. V., Lomakina, G. Y., Kotova, E. A., and Antonenko, Y. N. (2020) Bicarbonate suppresses mitochondrial membrane depolarization induced by conventional uncouplers, Biochem. Biophys. Res. Commun., 530, 29-34, https://doi.org/10.1016/j.bbrc.2020.06.131.
Funding
This work was financially supported by the Russian Science Foundation (project no. 19-14-00173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zorov, D.B., Andrianova, N.V., Babenko, V.A. et al. Nonphosphorylating Oxidation in Mitochondria and Related Processes. Biochemistry Moscow 85, 1570–1577 (2020). https://doi.org/10.1134/S0006297920120093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920120093