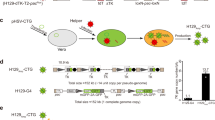
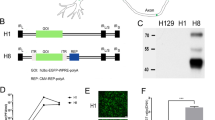
Abstract
Anterograde viral tracers are powerful and essential tools for dissecting the output targets of a brain region of interest. They have been developed from herpes simplex virus 1 (HSV-1) strain H129 (H129), and have been successfully applied to map diverse neural circuits. Initially, the anterograde polysynaptic tracer H129-G4 was used by many groups. We then developed the first monosynaptic tracer, H129-dTK-tdT, which was highly successful, yet improvements are needed. Now, by inserting another tdTomato expression cassette into the H129-dTK-tdT genome, we have created H129-dTK-T2, an updated version of H129-dTK-tdT that has improved labeling intensity. To help scientists produce and apply our H129-derived viral tracers, here we provide the protocol describing our detailed and standardized procedures. Commonly-encountered technical problems and their solutions are also discussed in detail. Broadly, the dissemination of this protocol will greatly support scientists to apply these viral tracers on a large scale.






Similar content being viewed by others
Abbreviations
- HSV-1:
-
Herpes simplex virus 1
- H129:
-
Herpes simplex virus 1 (HSV-1) strain H129
- tdT:
-
tdTomato
- fMOST:
-
Fluorescence Micro-Optical Sectioning Tomography
- TK:
-
Thymidine kinase
- AAV:
-
Adeno-associated virus
- CTB:
-
Cholera Toxin B
- MOI:
-
Multiplicity of infection
- CPE:
-
Cytopathic effects
- FBS:
-
Fetal bovine serum
- PBS:
-
Phosphate-buffered saline
- Pen–strep:
-
Penicillin–streptomycin
- PFA:
-
Paraformaldehyde
- OB:
-
Olfactory bulb
- M1:
-
Primary motor cortex
- Pir:
-
Piriform cortex
- LEnt:
-
Lateral entorhinal cortex
- Ent:
-
Entorhinal cortex
- CA1:
-
Field CA1 of the hippocampus
- BLA:
-
Basolateral amygdala
References
Nassi JJ, Cepko CL, Born RT, Beier KT. Neuroanatomy goes viral! Front Neuroanat 2015, 9: 80.
Schultz BR, Chamberlain JS. Recombinant adeno-associated virus transduction and integration. Mol Ther 2008, 16: 1189–1199.
Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods 2000, 103: 63–71.
Wickersham IR, Sullivan HA, Seung HS. Axonal and subcellular labelling using modified rabies viral vectors. Nat Commun 2013, 4: 2332.
Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci 1995, 15: 2972–2984.
Garner JA, LaVail JH. Differential anterograde transport of HSV type 1 viral strains in the murine optic pathway. J Neurovirol 1999, 5: 140–150.
McGovern AE, Davis-Poynter N, Farrell MJ, Mazzone SB. Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience 2012, 207: 148–166.
McGovern AE, Davis-Poynter N, Rakoczy J, Phipps S, Simmons DG, Mazzone SB. Anterograde neuronal circuit tracing using a genetically modified herpes simplex virus expressing EGFP. J Neurosci Methods 2012, 209: 158–167.
Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci 2004, 24: 2782–2786.
Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 2009, 296: R501–511.
Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol 2012, 302: R1049–1058.
Sun N, Cassell MD, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol 1996, 70: 5405–5413.
Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 2009, 29: 14223–14235.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 2003, 23: 8432–8444.
Lo L, Anderson DJ. A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 2011, 72: 938–950.
Zeng WB, Jiang HF, Gang YD, Song YG, Shen ZZ, Yang H, et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol Neurodegener 2017, 12: 38.
Etessami R, Conzelmann KK, Fadai-Ghotbi B, Natelson B, Tsiang H, Ceccaldi PE. Spread and pathogenic characteristics of a G-deficient rabies virus recombinant: an in vitro and in vivo study. J Gen Virol 2000, 81: 2147–2153.
Chen YH, Keiser MS, Davidson BL. Adeno-associated virus production, purification, and titering. Curr Protoc Mouse Biol 2018, 8: e56.
Dong X, Zhou J, Qin HB, Xin B, Huang ZL, Li YY, et al. Anterograde viral tracer herpes simplex virus 1 strain H129 transports primarily as capsids in cortical neuron axons. J Virol 2020, 94.
Tang H, Wu GS, Xie J, He X, Deng K, Wang H, et al. Brain-wide map of projections from mice ventral subiculum. Neurosci Lett 2016, 629: 171–179.
Deng K, Yang L, Xie J, Tang H, Wu GS, Luo HR. Whole-brain mapping of projection from mouse lateral septal nucleus. Biol Open 2019, 8.
Pi G, Gao D, Wu D, Wang Y, Lei H, Zeng W, et al. Posterior basolateral amygdala to ventral hippocampal CA1 drives approach behaviour to exert an anxiolytic effect. Nat Commun 2020, 11: 183.
Zhu X, Zhou W, Jin Y, Tang H, Cao P, Mao Y, et al. A central amygdala input to the parafascicular nucleus controls comorbid pain in depression. Cell Rep 2019, 29: 3847–3858 e3845.
Wang YY, Wang Y, Jiang HF, Liu JH, Jia J, Wang K, et al. Impaired glutamatergic projection from the motor cortex to the subthalamic nucleus in 6-hydroxydopamine-lesioned hemi-parkinsonian rats. Exp Neurol 2018, 300: 135–148.
Blaho JA, Morton ER, Yedowitz JC. Herpes simplex virus: propagation, quantification, and storage. Curr Protoc Microbiol 2005, Chapter 14: Unit 14E 11.
Services USDH, Control CfD, Prevention, Health Do, Services H, Health NIo. Biosafety in Microbiological and Biomedical Laboratories. Createspace Independent Pub, 2013.
Yu K, Ahrens S, Zhang X, Schiff H, Ramakrishnan C, Fenno L, et al. The central amygdala controls learning in the lateral amygdala. Nat Neurosci 2017, 20: 1680–1685.
Li Y, Xu J, Liu Y, Zhu J, Liu N, Zeng W, et al. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat Neurosci 2017, 20: 559–570.
Jin Y, Meng Q, Mei L, Zhou W, Zhu X, Mao Y, et al. A somatosensory cortex input to the caudal dorsolateral striatum controls comorbid anxiety in persistent pain. Pain 2020, 161: 416–428.
Keiser MS, Chen YH, Davidson BL. Techniques for intracranial stereotaxic injections of adeno-associated viral vectors in adult mice. Curr Protoc Mouse Biol 2018, 8: e57.
Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Elsevier Academic Press, 2004.
Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp 2012, 65: 3564.
Wang H, Zhu Q, Ding L, Shen Y, Yang CY, Xu F, et al. Scalable volumetric imaging for ultrahigh-speed brain mapping at synaptic resolution. Nat Sci Rev 2019, 6: 982–992.
Ma Q, Fu Y, Cao Z, Shao D, Song J, Sheng H, et al. A conditioning-strengthened circuit from CA1 of dorsal hippocampus to basolateral amygdala participates in morphine-withdrawal memory retrieval. Front Neurosci 2020, 14.
Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 2017, 93: 33–47.
Zhao F, Jiang HF, Zeng WB, Shu Y, Luo MH, Duan S. Anterograde trans-synaptic tagging mediated by adeno-associated virus. Neurosci Bull 2017, 33: 348–350.
Su P, Wang H, Xia J, Zhong X, Hu L, Li Y, et al. Evaluation of retrograde labeling profiles of HSV1 H129 anterograde tracer. J Chem Neuroanat 2019, 100: 101662.
Wojaczynski GJ, Engel EA, Steren KE, Enquist LW, Patrick Card J. The neuroinvasive profiles of H129 (herpes simplex virus type 1) recombinants with putative anterograde-only transneuronal spread properties. Brain Struct Funct 2015, 220: 1395–1420.
Acknowledgements
We appreciate Prof. Bo Li (Cold Spring Harbor Laboratory, USA), Xiaohui Zhang (Beijing Normal University, China), Xiao-Min Wang (Capital Medical University, China), Jian-Zhi Wang (Huazhong University of Science and Technology, China), Zhi Zhang (University of Science and Technology of China, China), and Ping Zheng (Fudan University, China) for providing the tracing parameters and results. We would like to thank the Animal Experiment Center and Core Facilities and Analytical Center of the Wuhan Institute of Virology for their support in animal experiments and imaging. This work was supported by the National Key Basic Research Program of China (2015CB755601), the National Natural Science Foundation of China (81427801, 81571355, and 81601206), and the National Institutes of Health RF1 Funding of China (RF1MH120020-01).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim that there are no conflicts of interest. The Wuhan Institute of Virology has filed a patent application for H129-G4 (US Patent No. 201615747742), and a patent application for H129-dTK-tdT and H129-dTK-T2 is still pending. The authors declare no other competing interests.
Ethics Approval and Consent to Participate
The standards of performance and animal studies were approved by the Institutional Review Board and Institutional Animal Welfare Committee (WIVA10201502), including intracerebral inoculation of mice and rats with viral tracers. All the experiments with viruses were performed in bio-safety level 2 laboratory and animal facilities.
Rights and permissions
About this article
Cite this article
Yang, H., Xiong, F., Song, YG. et al. HSV-1 H129-Derived Anterograde Neural Circuit Tracers: Improvements, Production, and Applications. Neurosci. Bull. 37, 701–719 (2021). https://doi.org/10.1007/s12264-020-00614-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00614-3