Abstract
Background
The removal of phenol from aqueous solution via photocatalytic degradation has been recognized as an environmentally friendly technique for generating clean water. The composite nanofibers containing PAN polymer, CNT, and TiO2 NPs were successfully prepared via electrospinning method. The prepared photocatalyst is characterized by SEM, XRD, and Raman spectroscopy. Different parameters are studied such as catalyst amount, the effect of pH, phenol concentration, photodegradation mechanism, flow rate, and stability of the composite nanofiber to evaluate the highest efficiency of the photocatalyst.
Results
The composite nanofibers showed the highest photodegradation performance for the removal of phenol using UV light within 7 min. The pH has a major effect on the photodegradation of phenol with its maximum performance being at pH 5.
Conclusions
Given the stability and flexibility of the composite nanofibers, their use in a dynamic filtration is possible and can be even reused after several cycles.
Similar content being viewed by others
Background
The contamination of water resources is a serious environmental concern. The water quality has been constantly decreasing due to the existence of different pollutants such as dyes, synthetic hormones, phenol, and pharmaceuticals [1, 2]. Phenol and phenolic compounds are one of the most persistent toxic organic pollutants discharged in wastewater effluents, which are resistant to environmental degradation through chemical, biological, and photolytic processes [3, 4]. The major sources of phenol and phenolic compounds are discharges of chemical process industries such as pulp and paper, pharmaceutical, agrochemical, petrochemical, and pesticide production [5]. The accumulation of these pollutants can have adverse effects on human health due to their toxicity, their ability to disrupt the endocrine system and their carcinogenic behavior [6]. Therefore, the improper handling and disposal of these toxic carcinogenic compounds pose a significant risk to the environment and the ecosystem [7, 8]. In order to deal with such pollutants, conventional adsorption technologies, such as biological degradation, chemical oxidation, adsorption, and photocatalysis are used for removal procedures [9, 10].
In recent years, photocatalysis has become an alternative for the removal of organic pollutants, including the treatment of phenolic wastewater s due to its economic, efficient, and green feature [11, 12]. Photocatalysis has many advantages including its use in UV, visible light radiation, and no waste because of its complete mineralization at the end of disintegration [13]. In addition, organic pollutants decompose in the presence of a wideband gap semiconductor which can promote reactions in the existence of UV light without being consumed in the entire reaction.
TiO2 is one of the most active catalysts among the semiconductors for the treatment of organic and contaminated components, due to its photosensitivity, low price, biological activity, and chemical stability [14, 15]. In practical applications, it is necessary to recover the remaining TiO2 nanoparticles in the photocatalytic reaction solution. In addition, carbon nanotube (CNT) has received a lot of attention as adsorbent due to their large specific surface area, small size, high porosity, and light mass density [16, 17]. The combining of TiO2 with CNTs has shown a major effect that can improve the overall performance of the photocatalytic process [15, 18]. TiO2 is actually an n-type semiconductor, however, in the existence of CNTs, photogenerated electrons can move freely towards to the surface of CNTs surface, which may have a lower Fermi level and leave too much valence band holes in the TiO2 to migrate to the surface and react [19, 20]. Also, CNTs can provide TiO2 with space restriction and a larger contact area, which leads to faster observed redox reaction rate [21]. Further, the application of anodic potentials to irradiated TiO2/CNTs composite can be further improved [22, 23]. The uniformity of the oxide coating and the physical properties of the composite materials vary depending on the manufacturing processes. In this regard, composite nanofibers have been used to immobilize TiO2/CNTs on the nanofibers [24, 25].
The hybrid photocatalysis and nanofiber membrane combines the advantages of both, membrane filtration and photocatalytic degradation. This approach allows the possibility of creating composite nanofibers membranes with superior removal efficiency and selectivity that lead to the development of a new water treatment solution [26, 27]. Given its facility of control and environmental sustainability, electrospinning is a versatile and effective process for the production of composite nanofibers with diameters from nanometers to submicrometers and characterized by their low cost, ease of use, and unique properties [28]. Electrospun PAN can be a promising carrier for immobilized catalytic materials since electrospun nanofibers based on PAN are hydrophobic, have a low density and are flexible in operation, thereby ensure easy floating on liquid or fixing at the desired location in the reactors [29, 30]. The above considerations form the basis of our further work. Herein, we investigate the utilization of a PAN-CNT/TiO2-NH2 nanofiber membrane in the application of organic wastewater decontamination. In this paper, the properties of PAN-CNT/TiO2-NH2 composite nanofibers by SEM, XRD, Raman spectra, and UV–Vis were investigated. The effect of catalyst dosage, contact time, pH solution, photodegradation mechanism, flow rate, and phenol concentration on the photocatalytic degradation of phenol under UV light are presented.
Materials and methods
Chemicals and materials
Analytical-grade chemicals used in this work were purchased from Sigma-Aldrich Sweden Ltd. PAN (MW = 150,000), Aeroxide TiO2, 3-aminopropyltriethoxysilane, glutaraldehyde, polyacrylonitrile, dimethylformamide (DMF), sodium hydroxide and hydrochloric acid were used in the experimental studies. The variation of pH in the feed solutions was maintained by adding 0.1 M HCl and 0.1 M NaOH. MWCNTs were synthesized following the procedure described in Yousef et.al [31]. Phenol (99.0%) was used to make standards and aqueous solutions for photocatalytic reactions.
Material preparation
10 wt. % of PAN was dissolved in DMF and stirred for 5 h. The, 1 wt. % of CNT was added to the PAN/DMF solution. The mixed solution was stirred for an additional 24 h and used for nanofiber preparation. For electrospun nanofibers, 1 mL/h flow rate and 25 kV was required. The modified surfaces of TiO2 NPs were described in our previous work. A mixture of 0.5 g of TiO2 and 10 mL deionized water were mixed to facilitate the adsorption of the hydroxyl group. The TiO2 were dispersed in 100 ml of toluene via ultrasonication for 30 min. Then 3 ml of the silane was added to the solution. The suspension was refluxed at 110 °C for 24 h creating NH2 functional groups on the titanium dioxide. The nanofibers were then immersed in a crosslinking medium composed of 100 mL distilled water and 2.5 wt% GA and then shaken for 24 h.
Material characterization
The surface morphology of the composite nanofibers were characterized by field emission scanning electron microscope (FE-SEM, S-5000, Hitachi, Japan) using an acceleration voltage of 10 kV. The crystal structure of the samples was determined via X-ray diffraction (XRD) patterns, which was recorded with a Bruker D8-Advance using Cu-Ka radiation at a step time of 0.2 s per point in the range of 20–80°. Raman spectra (Thermo scientific, DXR) were recorded between 0 and 2000 cm−1 with 514 nm wavelength. The UV–Vis absorption spectrum of the phenol samples were recorded after irradiation in a wavelength range of 200–500 nm with a UV–visible spectrophotometer (Lambda 35, PerkinElmer).
Photocatalysis experiments
The photocatalytic degradation experiments of phenol were carried out under dynamic conditions of a flow rate of 7 ml/min using ultraviolet (UV) radiation as shown in Fig. 1. The photoreactor is a cylindrical pyrex-glass column with a capacity of 250 ml, an inner diameter of 20 mm, and a height of 300 mm. A UV-A lamp (315–400 nm) was used as a UV light source with an intensity of 100 W. The nanofiber mat (3 × 6 cm, 50 mg) was immersed in the phenol solution with the concentration of 10 mg/L before each experiment. The suspension was kept in the dark for 30 min to achieve the equilibrium on the surfaces of the composite nanofibers. After that, the sample was exposed to UV light; reference samples were collected for analysis. At a given interval time, 3 mL suspension was collected and analyzed using a UV–visible spectrophotometer. All experiments were duplicated to assure the consistency and reproducibility of the results. The effect of initial phenol concentration (10–100 mg/L) and pH values from (2–9) were examined. The photodegradation efficiency of phenol can be expressed as follows:
where Ci and C0 (mg/L) are the initial and equilibrium concentration of phenol. The filtration performance of the composite nanofiber membranes was also characterized. After pre-wetting the nanofibers mat with DI water for 1 h, the membrane was fixed in the photoreactor. The pure water flux was calculated at various flow rates as follows:
where mP is the mass of permeate (g), t is the filtration time, Am is the area of the composite membrane (cm2), and ρw is the density of water (g/cm3).
Results and discussion
To confirm the successful crosslinking process, preparation, and immobilization of NPs loaded on the nanofiber, SEM, XRD, and Raman spectra were carried out. Figure 2a and b shows the surface morphologies of the PAN-CNT and PAN-CNT/TiO2-NH2 composite nanofibers. It was clearly observed that the composite nanofiber membrane was smooth and uniform and crosslinked well with the NPs on the top surface of the nanofiber. The XRD analysis of PAN-CNT/TiO2-NH2 composite nanofiber is presented in Fig. 2c, proving the highly crystalline character of the TiO2 and CNTs nanoparticles. In addition, Fig. 2d shows the Raman spectra of the PAN, CNT, TiO2, and PAN-CNT/TiO2-NH2 composite nanofibers. The result shows that the composite nanofibers contain all the characteristic peaks of PAN, CNT, and TiO2, confirming the successful fabrication of the composite nanofiber membrane [32, 33].
Photocatalytic performance of composites nanofibers
To maximize the degradation efficiency, catalyst dose was found to be the potential catalyst for the degradation of phenol [34]. In order to explore its effective concentration, reactions were carried out with different catalyst quantities (TiO2-NH2) ranging from 5 to 30 mg and the amount of CNT fixed at 3 mg at 10 mg/L concentrations of phenol. At higher TiO2-NH2 the catalytic performance of the composite nanofibers is much better for the same photocatalytic time as shown in Fig. 3. The 20 mg catalyst showed the best photodegradation efficiency in 7 min. This behavior is due to the increase in the number of active sites and the high surface area of the nanofiber [35]. Therefore, the increase in electron–hole pairs on the catalyst surface leads to higher amounts of reactive hydroxyl radicals, which can be attributed to better degradation. The increased loading with CNT/TiO2-NH2 provides more binding sites for substrate molecules to adsorb on the catalyst surface [36].
Furthermore, the pH of the system exerts a profound influence on adsorption and absorption due to its influence on the surface properties of the adsorbent and ionization/dissociation of the ion [37]. In this study, the pH value range between 2 and 9 was explored, as shown in Fig. 4. Apparently, after 7 min of UV irradiation, the degradation efficiency at pH 5 is about 99.2% and drops to 85% at pH 9. The photodegradation efficiency at pH 2 is slightly lower than at pH 6, which is due to the potential dissociation of PAN-CNT/TiO2-NH2 composite nanofiber and the Cl− ions that generated through pH adjustment compete with phenol for adsorption, thereby reducing adsorption and photocatalytic performance under strongly acidic conditions [38]; whereas, at high alkaline, the photodegradation performance is highly lower. The variation in the photodegradation efficiency with pH can be attributed to the strong electrostatic interaction between phenol and TiO2 at a neutral state [39].
The suitable condition for the maximum degradation of 10 mg/L phenol was finalized by optimizing various reaction parameters. The effect of phenol concentration on degradation efficiency was performed by increasing the concentration from 10 mg/L to 100 mg/L (Fig. 5). The result shows that at pH 5 the photodegradation efficiency decreased with increasing phenol concentration from 10 to 100 mg/L. The photodegradation efficiency at 100 mg/L was 60% in less than 10 min. This behavior may be attributed to the fact that due to the increased phenol concentration, the energy of the UV light is intercepted before reacting with the photocatalyst.
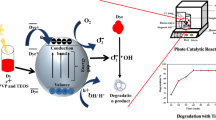
Figure 6 shows the individual components of the composite nanofiber to evaluate the photodegradation mechanism of phenol. The photocatalytic degradation efficiency of PAN/TiO2-NH2 and PAN-CNT was 79 and 45%, respectively. These results indicate that the photodegradation performance of phenol over the PAN-CNT/TiO2-NH2 composite nanofiber is more than PAN/TiO2-NH2 which can be attributed to that CNTs play an important role in improving the photodegradation efficiency and the increasing the number of active sites and the adsorption strength of each active site. The photodegradation mechanism of phenol using the composite nanofiber depends on the active species OH which is the key to the degradation process of organic substrates. To initiate the photoreaction, TiO2 is irradiated with energy equal or higher than its band gap. The photogenerated electrons are raised to the conduction band (CB) and photogenerated holes remain in the valence band (VB) [45]. The positively charged electron hole (h +) reacts with water to form (•OH) as shown in Fig. 7. Meanwhile the excited electron reduces an oxygen molecule to (O2•−) for degrading phenol to small molecules [46]. In addition, several researchers proposed that photocatalyst attack the hydroxyl radicals on the phenyl ring, resulting in the formation of catechol, then further oxidizing to oxalic acid and mineralizing to CO2 and H2O.
The reusability and stability performance of photocatalytic composite nanofibers is highly attractive for practical applications. Therefore, the photocatalytic degradation was performed for 3 cycles of photodegradation at 10 mg/L and pH 5 for 15 min under UV irradiation. Figure 8 shows its reusability and stability performance. The result showed that no significant decrease could be observed in the catalytic performance of the composite nanofiber membrane after three recycle experiments. This reveals that the catalyst is stable for more than 3 cycles and can be easily recycled for further use, which gives it a great potential in practical water treatment.
The pure water flux of the composite nanofiber membrane was tested as a function of the flow rate as shown in Fig. 9. The results show that the flux increases linearly with the flow rate. In general, the high flux of the composite nanofiber membrane is strongly desired as it consumes less energy and has high throughput which are the most important advantages pursued.
Conclusions
In summary, PAN-CNT/TiO2-NH2 composite nanofibers were successfully prepared via electrospinning processes for photocatalytic degradation of phenol by UV light. The composite nanofibers have stable performance and high photodegradation for phenol under UV light; nearly 99% within 7 min. This is attributed to the large surface area of the composite nanofiber which leads to significant improvement in light absorption, thereby achieving accelerated photodegradation and improving the photodegradation efficiency. The best result was achieved at the optimal condition of 20 mg catalyst, 10 mg/L phenol in neutral pH which is 5, and 7 min. In addition, the composite nanofibers are stable and reusable more than three times, which is an attractive performance for practical applications. Therefore, the present photocatalytic composite nanofiber membrane is an attractive option for large-scale environmental purification. Furthermore, compared to conventional nano-sized powder photocatalytic materials, it can be easily separated from the filtration system after photocatalytic reaction and reused more easily.
Availability of data and materials
Not applicable.
Abbreviations
- TiO2 :
-
Titanium dioxide
- CNT:
-
Carbon nanotube
- PAN:
-
Polyacrylonitrile
References
Hashem T, Ibrahim AH, Wöll C, Alkordi MH (2019) Grafting zirconium-based metal-organic framework UiO-66-NH2 nanoparticles on cellulose fibers for the removal of Cr(VI) ions and methyl orange from water. ACS Appl Nano Mater 2:5804–5808
A. Mohamed, Chapter Eight - Synthesis, Characterization, and Applications Carbon Nanofibers, in: S. Yaragalla, R. Mishra, S. Thomas, N. Kalarikkal, H.J. Maria (Eds.) Carbon-Based Nanofillers and Their Rubber Nanocomposites, Elsevier, 2019, pp. 243–257.
Grabowska E, Reszczyńska J, Zaleska A (2012) RETRACTED: Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: a review. Water Res 46:5453–5471
Mohamed A, Nasser WS, Kamel BM, Hashem T (2019) Photodegradation of phenol using composite nanofibers under visible light irradiation. Eur Polymer J 113:192–196
Malato S, Fernández-Ibáñez P, Maldonado MI, Blanco J, Gernjak W (2009) Decontamination and disinfection of water by solar photocatalysis: recent overview and trends. Catal Today 147:1–59
Aboamera NM, Mohamed A, Salama A, Osman TA, Khattab A (2017) Characterization and mechanical properties of electrospun cellulose acetate/graphene oxide composite nanofibers. Mech Adv Mater Struct 1:1–5
Swan SH (2008) Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 108:177–184
Yazdi MG, Ivanic M, Mohamed A, Uheida A (2018) Surface modified composite nanofibers for the removal of indigo carmine dye from polluted water. RSC Adv 8:24588–24598
Mohamed A, Osman TA, Toprak MS, Muhammed M, Uheida A (2017) Surface functionalized composite nanofibers for efficient removal of arsenic from aqueous solutions. Chemosphere 180:108–116
Mohamed A, Ghobara MM, Abdelmaksoud MK, Mohamed GG (2019) A novel and highly efficient photocatalytic degradation of malachite green dye via surface modified polyacrylonitrile nanofibers/biogenic silica composite nanofibers. Sep Purif Technol 210:935–942
Sajjad S, Leghari SA, Chen F, Zhang J (2010) Bismuth-doped ordered mesoporous TiO2: visible-light catalyst for simultaneous degradation of phenol and chromium. Chemistry (Weinheim an der Bergstrasse, Germany) 16:13795–13804
Khalil A, Aboamera NM, Nasser WS, Mahmoud WH, Mohamed GG (2019) Photodegradation of organic dyes by PAN/SiO2-TiO2-NH2 nanofiber membrane under visible light. Sep Purif Technol 224:509–514
Mohamed A, Salama A, Nasser WS, Uheida A (2018) Photodegradation of Ibuprofen, Cetirizine, and Naproxen by PAN-MWCNT/TiO2–NH2 nanofiber membrane under UV light irradiation. Environ Sci Europe 30:47
Uheida A, Mohamed A, Belaqziz M, Nasser WS (2019) Photocatalytic degradation of Ibuprofen, Naproxen, and Cetirizine using PAN-MWCNT nanofibers crosslinked TiO2-NH2 nanoparticles under visible light irradiation. Sep Purif Technol 212:110–118
Mohamed A, Osman TA, Toprak MS, Muhammed M, Yilmaz E, Uheida A (2016) Visible light photocatalytic reduction of Cr(VI) by surface modified CNT/titanium dioxide composites nanofibers. J Mol Catal A Chem 424:45–53
Mohamed A, Yousef S, Abdelnaby MA, Osman TA, Hamawandi B, Toprak MS, Muhammed M, Uheida A (2017) Photocatalytic degradation of organic dyes and enhanced mechanical properties of PAN/CNTs composite nanofibers. Separ Purif Technol. 182:219–223
Mohamed A, Osman TA, Khattab A, Zaki M (2014) Tribological behavior of carbon nanotubes as an additive on lithium grease. J Tribol 137:011801–011801
Salama A, Mohamed A, Aboamera NM, Osman T, Khattab A (2018) Characterization and mechanical properties of cellulose acetate/carbon nanotube composite nanofibers. Adv Polym Technol 37:2446–2451
Nam Y, Lim JH, Ko KC, Lee JY (2019) Photocatalytic activity of TiO2 nanoparticles: a theoretical aspect. J Mater Chem A 7:13833–13859
Leary R, Westwood A (2011) Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 49:741–772
Nguyen KC, Ngoc MP, Nguyen MV (2016) Enhanced photocatalytic activity of nanohybrids TiO2/CNTs materials. Mater Lett 165:247–251
Azzam EMS, Fathy NA, El-Khouly SM, Sami RM (2019) Enhancement the photocatalytic degradation of methylene blue dye using fabricated CNTs/TiO2/AgNPs/Surfactant nanocomposites. J Water Process Eng 28:311–321
Zhang W, Li G, Liu H, Chen J, Ma S, An T (2019) Micro/nano-bubble assisted synthesis of Au/TiO2@CNTs composite photocatalyst for photocatalytic degradation of gaseous styrene and its enhanced catalytic mechanism. Environ Sci Nano 6:948–958
Salama A, Mohamed A, Aboamera NM, Osman TA, Khattab A (2018) Photocatalytic degradation of organic dyes using composite nanofibers under UV irradiation. Appl Nanosci 8:155–161
Mohamed A, Nasser WS, Osman TA, Toprak MS, Muhammed M, Uheida A (2017) Removal of chromium (VI) from aqueous solutions using surface modified composite nanofibers. J Colloid Interface Sci 505:682–691
Abdel-Mottaleb MM, Mohamed A, Karim SA, Osman TA, Khattab A (2018) Preparation, characterization, and mechanical properties of polyacrylonitrile (PAN)/graphene oxide (GO) nanofibers. Mech Adv Mater Struct 1:1–6
Karim SA, Mohamed A, Abdel-Mottaleb MM, Osman TA, Khattab A (2019) Visible light photocatalytic activity of PAN-CNTs/ZnO-NH2 electrospun nanofibers. J Alloy Compd 772:650–655
Abdel-Mottaleb MM, Khalil A, Karim S, Osman TA, Khattab A (2019) High performance of PAN/GO-ZnO composite nanofibers for photocatalytic degradation under visible irradiation. J Mech Behav Biomed Mater 96:118–124
Abdel-Mottaleb MM, Khalil A, Osman TA, Khattab A (2019) Removal of hexavalent chromium by electrospun PAN/GO decorated ZnO. J Mech Behav Biomed Mater 98:205–212
Karim SA, Mohamed A, Abdel-Mottaleb MM, Osman TA, Khattab A (2018) Mechanical properties and the characterization of polyacrylonitrile/carbon nanotube composite nanofiber. Arabian J Sci Eng 43:4697–4702
Yousef S, Mohamed A (2016) Mass production of CNTs using CVD multi-quartz tubes. J Mech Sci Technol 30:5135–5141
Wang B, Xin H, Li X, Cheng J, Yang G, Nie F (2014) Mesoporous CNT@TiO2-C nanocable with extremely durable high rate capability for lithium-ion battery anodes. Sci Rep 4:3729
Khalil A, Nasser WS, Osman TA, Toprak MS, Muhammed M, Uheida A (2019) Surface modified of polyacrylonitrile nanofibers by TiO2/MWCNT for photodegradation of organic dyes and pharmaceutical drugs under visible light irradiation. Environ Res 179:108788
Saud PS, Pant B, Alam A-M, Ghouri ZK, Park M, Kim H-Y (2015) Carbon quantum dots anchored TiO2 nanofibers: Effective photocatalyst for waste water treatment. Ceram Int 41:11953–11959
Khalil AM, Schäfer AI (2021) Cross-linked β-cyclodextrin nanofiber composite membrane for steroid hormone micropollutant removal from water. J Membr Sci 618:118228
Ameta R, Solanki MS, Benjamin S, Ameta SC (2018) Chapter 6 – Photocatalysis. In: Ameta SC, Ameta R (eds) Advanced Oxidation Processes for Waste Water Treatment. Academic Press, New York, pp 135–175
Aboamera NM, Mohamed A, Salama A, Osman TA, Khattab A (2018) An effective removal of organic dyes using surface functionalized cellulose acetate/graphene oxide composite nanofibers. Cellulose 25:4155–4166
Najam-Khan M, Dutta J (2015) Comparison of photocatalytic activity of zinc stannate particles and zinc stannate/zinc oxide composites for the removal of phenol from water, and a study on the effect of pH on photocatalytic efficiency. Mater Sci Semicond Process. 36:124–133
Mohamed A, El-Sayed R, Osman TA, Toprak MS, Muhammed M, Uheida A (2016) Composite nanofibers for highly efficient photocatalytic degradation of organic dyes from contaminated water. Environ Res 145:18–25
Acknowledgement
T. Hashem acknowledges support through the Cluster “3DMM2O” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany`s Excellence Strategy – 2082/1 – 390761711.
Funding
Open Access funding enabled and organized by Projekt DEAL. Cluster “3DMM2O” funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany`s Excellence Strategy – 2082/1 – 390761711.
Author information
Authors and Affiliations
Contributions
AM: conceptualization, methodology, investigation, writing—original draft, writing—review and editing. SY: review and editing. WN: writing—original draft, analysis. TO, AK, EV, TH: review and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, A., Yousef, S., Nasser, W.S. et al. Rapid photocatalytic degradation of phenol from water using composite nanofibers under UV. Environ Sci Eur 32, 160 (2020). https://doi.org/10.1186/s12302-020-00436-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12302-020-00436-0