Abstract
Soil organic matter (SOM) in tropical forests is an important store of carbon (C) and nutrients. Although SOM storage could be affected by global changes via altered plant productivity, we know relatively little about SOM stabilisation and turnover in tropical forests compared to temperate systems. Here, we investigated changes in soil C and N within particle size fractions representing particulate organic matter (POM) and mineral-associated organic matter (MAOM) after 13 years of experimental litter removal (L−) and litter addition (L+) treatments in a lowland tropical forest. We hypothesized that reduced nitrogen (N) availability in L− plots would result in N-mining of MAOM, whereas long-term litter addition would increase POM, without altering the C:N ratio of SOM fractions. Overall, SOM-N declined more than SOM-C with litter removal, providing evidence of N-mining in the L− plots, which increased the soil C:N ratio. However, contrary to expectations, the C:N ratio increased most in the largest POM fraction, whereas the C:N ratio of MAOM remained unchanged. We did not observe the expected increases in POM with litter addition, which we attribute to rapid turnover of unprotected SOM. Measurements of ion exchange rates to assess changes in N availability and soil chemistry revealed that litter removal increased the mobility of ammonium-N and aluminium, whereas litter addition increased the mobility of nitrate-N and iron, which could indicate SOM priming in both treatments. Our study suggests that altered litter inputs affect multiple processes contributing to SOM storage and we propose potential mechanisms to inform future work.
Similar content being viewed by others
Introduction
Tropical forest soils play a crucial role in the global carbon (C) balance: they are the largest natural source of carbon dioxide (CO2) and contain almost 1/3 of global soil C stocks (Stockmann et al. 2013). Consequently, even small changes in soil C stocks or turnover rates in tropical forests have the potential to influence atmospheric CO2 concentrations. Global environmental changes such as warming, shifting precipitation patterns, and extreme weather events can influence soil C storage in tropical forests via their impacts on net primary productivity and plant litter inputs to the soil (Sayer et al. 2011). Plant litter can be considered the “pump” in the C cycle of terrestrial ecosystems, as the quality and quantity of litter inputs are key drivers of decomposition processes and soil organic matter formation (Paul 2016). Soil organic matter (SOM) can be regarded as a continuum of progressively decomposing organic compounds: much of the organic matter entering soils is recognisably plant material, which undergoes a series of biological, chemical and physical transformations into organic products that can be stabilised by association with soil minerals (Lehmann and Kleber 2015). Theoretically, increased plant inputs to the soil should promote SOM formation but experimental studies in temperate and tropical forests have clearly demonstrated that increased inputs of plant litter to the soil accumulate in relatively undecomposed fractions and do not necessarily result in larger pools of stabilised SOM, even over decadal timeframes (Sayer et al. 2019; Lajtha et al. 2014, 2018; Pisani et al. 2016).
Conceptually, SOM can be divided into particulate organic matter (POM), made up of relatively undecomposed lightweight fragments, and mineral-associated organic matter (MAOM) that is predominantly formed by the products of microbial decomposition (Lavallee et al. 2020; Cotrufo et al. 2019). The relative accumulation of POM and MAOM may be constrained by soil chemistry, microbial activity or nutrient availability, and also depends strongly on soil type and climate (Jilling et al. 2018; Cotrufo et al. 2019). In temperate soils, the persistence of MAOM is often attributed to associations with mineral matrices dominated by 2:1 phytosilicate clays, whereas in tropical soils, aluminium and iron oxides, oxyhydroxides and sesquioxides are thought to play a dominant role (Coward et al. 2017). By contrast, the persistence of POM is controlled mainly by microbial and enzymatic inhibition (Lavallee et al. 2020) and POM may therefore be a more sensitive indicator of changes to SOM formation (Lehmann et al. 2001). However, changes in POM accumulation and chemistry could modify POM turnover and subsequently affect the formation of MAOM, which will influence overall SOM stabilization and storage (Grandy and Neff 2008; Hofmockel et al. 2011).
Changes in nutrient availability, in particular nitrogen (N), can modify SOM stabilisation and storage and may differentially affect SOM associated with different fractions (Grandy et al. 2008). Nitrogen plays a key role in SOM formation (Billings and Ballantyne 2013) because the C:N ratio of organic matter in the early stages of decomposition is higher than the C:N ratio of the microbial biomass, and microbial decomposers therefore require N from supplemental sources (Hessen et al. 2004). Indeed, POM that has undergone little decomposition has a C:N ratio similar to plant material (c. 10–40; Fornara et al. 2011; Lavallee et al. 2020), which declines as decomposition progresses (Khan et al. 2016), resulting in MAOM with a C:N ratio similar to microbial biomass (c. 8–13; Giannetta et al. 2019; Lavallee et al. 2020). As the transformation of plant material into stabilised SOM requires N, the ability of soils to store C is strongly linked to N availability (Kirkby et al. 2013; Paul 2016; Cotrufo et al. 2019) and N limitation can reduce the microbial contribution to SOM formation (Khan et al. 2016). Furthermore, as MAOM is relatively N-rich compared to plant material, microbial degradation of MAOM can supply the N required for litter decomposition (Fahey et al. 2011; Chen et al. 2014). Consequently, progressive N limitation can constrain soil C storage under elevated atmospheric CO2, despite increased plant inputs to the soil (Hungate et al. 2003), whereas fertilization with N can reduce microbial degradation of MAOM (Hagedorn et al. 2003).
In highly weathered tropical soils, SOM stabilisation is often associated with high concentrations of iron (Fe) and aluminium (Al) oxides, which are capable of binding organic compounds into stable organo-mineral complexes (Coward et al. 2017). The formation of organo-mineral complexes by Al and Fe oxyhydroxides often involves nitrogenous compounds (Giannetta et al. 2019) which suggests that N availability might also play a key role in SOM stabilisation in tropical soils. Although N availability in lowland tropical soils is often high relative to other nutrients, tropical forest nutrient cycles are also characterized as efficient, and a large proportion of the nutrients required for plant growth is recycled in litterfall (Vitousek 1984). We have previously demonstrated that long-term experimental treatments removing or adding aboveground litter in a lowland tropical forest substantially altered the forest N status, whereby litter addition treatments resulted in a ‘leaky’ N cycle characterized by high concentrations of nitrate-N in the soil, and increased soil nitrous oxide emissions, whereas litter removal treatments reduced N concentrations in soil and plant material, resulting in enhanced plant N use efficiency (Sayer et al. 2020). Hence, our litter manipulation treatments have resulted in marked differences in the bioavailability of both C and N, which could result in decoupling of C and N dynamics during SOM formation (Bimüller et al. 2014). Indeed, substantial changes in plant litter inputs are likely to alter C and N cycling within SOM pools, or the transfer of C and N between pools (Hofmockel et al. 2011). Interestingly, both increased and reduced plant litter inputs could promote SOM mineralisation via ‘priming effects’, in which fresh organic matter stimulates the mineralisation and release of SOM (Kuzyakov et al. 2000). Disproportionate increases in soil respiration in response to litter addition treatments have been attributed to increased microbial activity in response to extra fresh organic matter (Sulzman et al. 2005; Crow et al. 2009; Sayer et al. 2007, 2011). However, declining N availability with long-term litter removal could also cause priming effects via microbial mining for N because N-deficient plants can increase root exudation to stimulate microbial release of N from SOM (Fontaine and Barot 2005; Fontaine et al. 2007; Bengtson et al. 2012; Dijkstra et al. 2013). In this case, MAOM may be particularly vulnerable to priming, as it constitutes an important source of N in the rhizosphere, with low C:N ratios that facilitate N mineralization (Jilling et al. 2018). Hence, changes in the distribution of C and N among SOM fractions (Hofmockel et al. 2011), and simultaneous evaluation of the size and C:N ratios of litter, microbial, and SOM pools could indicate whether altered litter inputs affect SOM storage by modifying N-availability.
Here, we quantified, for the first time, the C and N content and C:N ratios of different SOM fractions after more than a decade of monthly litter addition and litter removal treatments in a lowland tropical forest. Given that the litter manipulation treatments have substantially modified plant C inputs and altered forest N cycling, we aimed to assess whether differences in C and N inputs and availability between litter addition and removal treatments would alter the distribution of C and N among SOM fractions. Although we do not directly address the specific mechanisms underlying changes in SOM pools, we based the following hypotheses on substantially reduced N-availability with litter removal, and increased N cycling in the litter addition treatments (Sayer et al. 2020):
-
(H1)
The C and N content of SOM will have declined substantially in response to long-term litter removal, but reduced N availability will result in greater declines in N relative to C, and therefore higher SOM C:N ratios compared to controls
-
(H2)
As MAOM represents a substantial source of N, the largest declines in SOM-N with litter removal will be observed in MAOM fractions
-
(H3)
In litter addition treatments, high N availability and concurrent increases in C and N inputs with the added litter will result in increased POM C and N content, but no change in POM or MAOM C:N ratios.
We tested our hypotheses by quantifying the C and N concentrations in soil particle size fractions representing POM and MAOM. We determined the C and N content of the leaf litter and microbial biomass to assess resource availability to microbial decomposers, and we measured ion exchange rates to explore potential links between N-availability and MAOM stabilised by Al and Fe oxides.
Methods
Experimental design and sample collection
We characterised changes in carbon (C) and nitrogen (N) associated with different SOM fractions in a long-term litter manipulation experiment after 13 years of treatments. The study site is a 40-ha area of mature lowland tropical forest on Gigante Peninsula, part of the Barro Colorado Nature Monument, in Panama, Central America (9°06′N, 79°54′W). The soils at the study site are deep-red light clays (Baillie et al. 2007) classified as Oxisols (Cavelier 1992) or Ferralsols with kaolinite as the dominant clay mineral (Baillie et al. 2007). Soil pH is c. 5.5 and total organic C content is c. 4–5% at 0–10 cm depth (Sayer et al. 2012; 2019). The litter manipulation experiment comprises fifteen 45-m × 45-m plots where, starting in January 2003, the litter in five L− plots is removed each month and added to five L+ plots, nominally doubling litter inputs, leaving five plots as undisturbed controls (CT; Sayer and Tanner 2010; Sayer et al. 2020). Previous work within the experimental plots has revealed no differences in soil water content among treatments, and only minor changes in soil temperature (< 0.5 °C at 0–10 cm depth; Sayer et al. 2007).
After 13 years of treatments in September 2016, we took five soil samples at 0–10 cm depth in each plot using a 3-cm diameter punch corer. The soil cores were pooled to give one composite sample per plot and all subsequent analyses were performed on subsamples of the same soils (n = 5 per treatment). We focussed on mineral soil at 0–10 cm depth because we have previously shown no differences among treatments in soil organic C or total N at 10–20 cm or 20–30 cm depth (Tanner et al. 2016; Sayer et al. 2019). To determine litter C and N concentrations we sampled monthly litterfall collected in five traps per plot towards the end of the main growing season in October 2017. The woody fraction was excluded, and samples were pooled by plot, shredded and a subsample was finely ground for analysis. The concentrations of C and N in the litter were determined by complete combustion gas chromatography (Thermo Flash EA1112, CE, Elantech, Lakewood, NJ, USA).
Particle-size fractionation
We quantified SOM associated with four soil particle-size fractions. While we acknowledge that particle size fractionation does not definitively distinguish POM and MAOM, previous studies in the experimental plots demonstrated that particle size fractionation yielded similar results to density fractions (Cusack et al. 2018; Sayer et al. 2019). Hence, we use the cut-off points for POM vs. MAOM recommended by Lavallee et al. (2020) for ease of interpretation, whereby we refer to two size fractions representing POM (2000–200 µm and 200–50 µm) and two size fractions representing MAOM (50–20 µm and < 20 µm).
We performed particle-size fractionation by wet-sieving (Lopez-Sangil and Rovira 2013) on two analytical replicates of each soil sample as described in Sayer et al. (2019). Briefly, air-dried soil samples (c. 20 g) were agitated with distilled water (1:2 w:v) and glass beads for 60 min at 160 rpm to break up soil macro-aggregates. The soil slurry was passed through a 200-µm sieve, washed thoroughly and micro-aggregates were then disrupted by ultrasonication (55 W power and 440 J cm−3 energy outputs), which was optimised to the soils at the study site following recommendations to limit C transfer among fractions (Kaiser and Berher 2014; Poeplau and Don 2014). The resulting slurry was passed through a sequence of two sieves (50 and 20-µm mesh) into a receiver, and the material retained in the 200-µm sieve was passed through 2-mm mesh to remove large particles. The slurry retained in the receiver (particle size < 20 µm) was brought to 1 L volume with distilled water and resuspended with 8.7 g K2SO4 for overnight particle flocculation, after which the supernatant was siphoned off. All fractions were transferred to glass beakers, dried at 65 °C and weighed. Ground subsamples of the bulk soil and the four size fractions (2000–200; 200–50; 50–20 and < 20 µm) were analysed for total N and organic C content by combustion oxidation (Vario EL III CN analyser, Elementar Analyser Systems, Hanau, Germany). The recovery of C and N in the particle size fractions was 95 ± 2.5 and 94 ± 2.7%, respectively, and there were no significant differences in the proportion of C or N recovered among litter manipulation treatments.
Microbial biomass and extractable C and N
To assess how litter manipulation affected the stoichiometry of microbial decomposers and extractable SOM, we determined total microbial biomass carbon (CMIC) and nitrogen (NMIC) by chloroform fumigation extraction using paired subsamples of fresh soil (8 g fresh weight) following Vance et al. (1987) and Jones and Willett (2006). Briefly, one subsample was immediately extracted in 40 ml 0.5 M K2SO4 and the other sample was fumigated with ethanol-free chloroform for 24 h prior to extraction. All extracts were shaken for 1 h, filtered through pre-washed filter paper (Whatman® 42) and the total C and N content of the extracts were analysed by oxidation combustion (TOC-L, Shimadzu Corporation, Kyoto, Japan). CMIC and NMIC were then calculated as the difference between extractable C and N from fumigated and unfumigated subsamples without correction for extraction efficiency. We considered the C and N in the K2SO4 extracts of unfumigated samples as “extractable” C and N, which is highly bioavailable (Giannetta et al. 2019).
Exchange rates of soil inorganic N, iron and aluminium
To assess whether the availability of N to plants might explain changes in SOM fractions, we measured the exchange rates of ammonium-N and nitrate-N in the soil during the growing season using ion exchange resins (PRS Probes, Western Ag, Canada). Given the importance of organo-mineral complexes involving aluminium (Al) and iron (Fe) oxides in MAOM formation in tropical soils (Zech et al. 1997; Coward et al. 2017; Rasmussen et al. 2018), we also measured the exchange rates of Fe and Al as potential indicators of MAOM mobilisation. The exchange rates for nitrate-N, but not ammonium-N, and exchangeable base cations (K, Ca, Mg) have been previously reported elsewhere (Sayer et al. 2020). We buried four pairs of anion and cation exchange probes vertically (0–10 cm depth) in the soil in each plot for 42 days during the peak growing season (June–July) and again towards the end of the growing season (Nov–Dec) in 2017. After retrieval, the probes were cleaned with deionised water and returned to the manufacturer for analysis, where nitrate-N was determined by colourimetry and all remaining nutrient ions were measured by ICP-MS after acid digest. Nutrient exchange rates are based on the surface area of the resin and the exposure time, and thus units are given as μg nutrient 10 cm−2 42 d−1.
Data analyses
The C and N concentrations of different fractions were corrected for differences in bulk density as described in Sayer et al. 2019 and log-transformed as necessary to meet modelling assumptions; all statistical analyses were performed using mean values per plot (n = 5 per treatment) in R version 3.6.1 (R Core Team 2020). Although we previously omitted one plot per treatment due to a large tree-fall (Sayer et al. 2019), here we included the data from all 15 plots to retain statistical power despite contamination issues with the samples for total N concentrations and microbial biomass in one L− and one CT plot, respectively.
We investigated whether litter treatments affected the C and N concentrations or C:N ratio of SOM associated with different size fractions using linear mixed effects models (LME; lmer function in the lme4 package; Pinheiro and Bates 2000), with treatment, fraction, and their interaction as fixed effects and block as a random effect. We assessed changes in the relative proportions of POM vs. MAOM-C and N using Generalised Linear Models (GLM) with a quasi-binomial error distribution. We further tested differences among treatments in the C and N concentrations and C:N ratios of microbial biomass, K2SO4 extracts, leaf litter and bulk soil using one-way analysis of variance (ANOVA), or Kruskal–Wallis tests when data were resistant to transformation. Finally, we tested the effects of treatment on the exchange rates of ammonium-N, nitrate-N, Al and Fe using linear mixed effects models with treatment as a fixed effect, and time and block as random effects. Where overall treatment effects were significant, we compared individual treatment levels (litter addition or removal) to controls using post-hoc tests based on Satterthwaite’s approximation for linear mixed effects models (difflsmeans function in the lmerTest package; Kuznetsova et al. 2017), treatment contrasts for ANOVAs, and Holm’s post-hoc tests for Kruskal–Wallis tests (DunnTest function in the DescTools package; Signorell et al. 2018). We report significant effects at p < 0.05 and, given the potential relevance of small changes in SOM over time and the limited number of replicates (n = 5), we also report trends at p < 0.1.
Results
Bulk soil and particle size fractions
Litter removal substantially affected soil C and N, but the effect of litter addition was much smaller. Total soil organic C (TOC) was 37 ± 6% lower in the L− plots and 19 ± 8% higher in the L+ plots compared to the controls (Fig. 1a; F2,12 = 35.7, p < 0.001), whereas total N was significantly 43 ± 7% lower in the L− plots relative to the controls (F2,12 = 42.2, p < 0.001) and the slight increase in total N in the L+ plots was not significant (Fig. 1b). Overall, the decline in total N with litter removal was greater than the decline in TOC and there was a strong trend towards a higher soil C:N ratio in the L− plots (12.5 ± 0.3) relative to the controls (11.1 ± 0.2; F2,12 = 3.8, p = 0.053), but no change in the soil C:N ratio in the L+ plots (11.9 ± 0.5; Fig. 1c).
Differences in a total organic carbon (TOC) b total nitrogen (TN), and c the C:N ratio of the soil at 0–10 cm depth after 13 years of litter manipulation treatments in a lowland tropical forest in Panama, Central America, where L+ is litter addition (orange), L− is litter removal (purple) and CT is controls (blue); soil mass was corrected for differences in bulk density among treatments. Boxes denote the 25th and 75th percentiles and median lines are given for n = 5, whiskers indicate values up to 1.5× the interquartile range, and dots indicate outliers. (Color figure online)
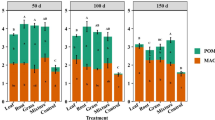
Most of the soil organic C was contained in MAOM (< 50 µm), which accounted for > 79% of the TOC across all treatments, whereas the C contained in POM (2000–50 µm) only accounted for 8–10% of the TOC. Litter manipulation altered the distribution of C between POM and MAOM: a smaller proportion of TOC (79 ± 6%) was contained in MAOM in the L+ plots compared to the controls (89 ± 2%; GLM: χ2 = 0.27, p = 0.002) but litter removal did not affect the proportion of TOC contained in MAOM (91 ± 3%). By contrast, a smaller proportion of TOC was contained in POM in the L− plots (8.0 ± 0.4%) compared to the controls (9.7 ± 0.9%; GLM: χ2 = 0.08, p = 0.045), whereas litter addition had no effect (9.1 ± 0.5%; Fig. 2a). The amount of MAOM-C was significantly lower in L− plots compared to controls but there was no effect of litter addition, and although POM-C had declined markedly in the L− plots compared to the L+ plots, neither treatment differed significantly from the controls (Table 1; Fig. 2b; LME: χ2 = 115.3, p < 0.001).
Differences in particulate organic matter (POM) and mineral-associated organic matter (MAOM) shown as a the proportion of total organic carbon (TOC); b carbon (C) concentration; c the proportion of total nitrogen (TN) and d nitrogen (N) concentration in the soil at 0–10 cm depth after 13 years of litter manipulation treatments in a lowland tropical forest in Panama, Central America. Symbols and abbreviations follow the legend to Fig. 1. Note that the y-axes differ between panels
The distribution of N in MAOM and POM largely mirrored that of C, whereby the N contained in MAOM accounted for > 83% of the total N across treatments and the N contained in POM accounted for only 3–4% of total N. Although the proportion of total N contained in MAOM and POM did not differ among treatments (Fig. 2c), the amounts of MAOM-N and POM-N were markedly lower in the L− plots compared to the controls, with the largest decline in POM-N (Table 1; Fig. 2d; χ2 = 116.2, p < 0.001). Although litter removal resulted in greater declines in N than C in both fractions (Table 1), the C:N ratios of MAOM (CT: 11.0 ± 0.3, L+ : 11.2 ± 0.3, L−: 11.7 ± 0.2) and POM (CT: 26 ± 1, L+ : 29 ± 2, L−: 30 ± 2) did not differ among treatments.
The distribution of C and N differed among particle size fractions, with the largest amount of C and N in the < 20 µm MAOM fraction and the smallest amount in the 200–50 µm POM fraction (Fig. 3). As expected, the C:N ratios of the fractions declined with particle size, with the highest and most variable C:N ratio in the 2000–200 µm POM fraction and the lowest C:N ratio in the < 20 µm MAOM fraction (Fig. 4). The distribution of C among size fractions was similar across treatments, although the C concentration in each fraction was generally higher in the L+ plots and lower in the L− plots compared to the controls (Table 1; Fig. 3a; LME, treatment + fraction effect: χ2 = 222.94, p < 0.001).
Differences in a carbon and b nitrogen content of soil particle size fractions at 0–10 cm depth after 13 years of litter manipulation treatments in a lowland tropical forest in Panama, Central America; particulate organic matter is represented by the 2000–200 and 200–50 µm fractions, and mineral-associated organic matter is represented by the 50–20 and < 20 µm fractions. Symbols and abbreviations follow the legend to Fig. 1. Note that the y-axes differ among panels
Changes in the carbon to nitrogen (C:N) ratios of soil particle size fractions at 0–10 cm depth after 13 years of litter addition (L+), litter removal (L−) and control (CT) treatments in a lowland tropical forest in Panama, Central America; particulate organic matter is represented by the 2000–200 and 200–50 µm fractions, and mineral-associated organic matter is represented by the 50–20 and < 20 µm fractions. Symbols follow the legend to Fig. 1
Litter manipulation altered the distribution of N among size fractions (Fig. 3b; treatment × fraction interaction; χ2 = 222.94, p < 0.001). The amount of N in all fractions was substantially lower in the L− plots than in the controls, whereas in the L+ plots, only the 200–50 µm POM fraction contained more N than the controls (Table 1; Fig. 3b). Consequently, litter removal increased the C:N ratios of the 2000–200 POM µm and 50–20 µm MAOM fractions, and litter addition increased the C:N ratio of the 2000–200 POM µm fraction relative to the controls, but the C:N ratio of the < 20 µm MAOM fraction did not differ among treatments (Fig. 4).
Extractable, microbial and litter C and N
Litter removal reduced the amounts of C or N in extractable SOM and microbial biomass, but there was no effect of litter addition. Extractable C was 35 ± 7% lower in L− plots compared to the controls, and extractable N was 44 ± 7% lower (Fig. 5a,b; Kruskal, χ2 = 6.72, p = 0.035 and χ2 = 9.5, p = 0.009, for C and N, respectively), whereas microbial C was 49 ± 15% lower in the L− plots compared to controls (ANOVA: F2,11 = 5.87, p = 0.017) but microbial N did not differ among treatments (Fig. 5a,b). However, litter addition reduced the C:N ratio of extractable SOM compared to the controls, whereas litter removal had no effect (Fig. 5c; ANOVA: F2,11 = 9.35, p = 0.004) and the C:N ratio of the microbial biomass was unaffected by litter manipulation (Fig. 5c). Litter C, N and C:N ratios did not differ between either treatment and the controls, although litter N was 18 ± 4% lower in L− compared to L+ plots (Fig. 5a,b) and consequently, the litter C:N ratio was slightly higher in the L− plots than in the L+ plots (Fig. 5c).
Differences in a carbon (C) and b nitrogen (N) concentrations, and c C:N ratios of K2SO4-extractable soil organic matter (extractable), microbial biomass (microbial) at 0–10 cm depth and mixed leaf litter (litter) after 13 years of litter addition (L+), litter removal (L−) and control (CT) treatments in a lowland tropical forest in Panama, Central America. Symbols follow the legend to Fig. 1. Note that the y-axes differ among panels
Nutrient exchange rates
Finally, litter manipulation altered the exchange rates of inorganic N, iron and aluminium in the soil during the growing season (Fig. 6). The exchange rates of ammonium-N were higher in the L− plots compared to the controls (LME: χ2 = 8.8, p = 0.012), indicating greater availability of ammonium-N, especially at the start of the growing season in June (Fig. 6a). By contrast, nitrate-N exchange rates were substantially higher in the L+ plots but lower in the L− plots compared to the controls at both the start and the end of the growing season (LME: χ2 = 20.1, p < 0.001; Fig. 6b). Interestingly, aluminium exchange rates were twice as high in the L− plots compared to controls (LME: χ2 = 20.9, p < 0.001; Fig. 6c), whereas iron exchange rates were twice as high in the L+ plots (LME: χ2 = 24.7, p < 0.001; Fig. 6d), which suggests that litter manipulation substantially altered the mobility of these metal ions in the soil.
Exchange rates of ammonium-N, nitrate-N, aluminium and iron in the soil at 0–10 cm depth in June and November 2017 after 14 years of litter addition (L+), litter removal (L−) and control (CT) treatments in a lowland tropical forest in Panama, Central America. Symbols follow the legend to Fig. 1. Note that the y-axes differ among panels
Discussion
Our long-term experimental treatments allowed us to assess the potential influence of litter addition and removal on SOM formation via changes in nutrient availability. Although the reduction in SOM after 13 years of litter removal in our forest was substantial, the gain in SOM with litter addition was minimal. We hypothesised that reduced N availability to plants as a result of long-term litter removal (Sayer et al. 2020) would promote microbial N-mining, resulting in higher SOM C:N ratios in the L− plots (H1), particularly in MAOM fractions (H2). By contrast, we expected that enhanced availability of C and N with litter addition would result in accumulation of POM in the L+ plots (H3). Overall, the declines in SOM-N pools with litter removal were generally greater than the declines in SOM-C pools (Table 1), resulting in a higher C:N ratio of the bulk soil and specific particle size fractions in the L− plots (Figs. 1, 4), which provides evidence to support our hypothesis that N-mining might contribute to the degradation of SOM. However, the decline in MAOM-N with litter removal was smaller than expected, and we found little evidence of POM accumulation with long-term litter addition. We discuss the evidence for microbial N-mining of SOM alongside the changes in SOM-C pools in response to 13 years of litter removal and addition treatments and propose potential mechanisms worthy of further investigation.
Evidence for microbial N-mining of SOM with litter removal
A prerequisite for N-mining is the availability of an energy-rich C source to microbial decomposers capable of degrading SOM to release N (Fontaine et al. 2011). Although our monthly litter removal treatments effectively eliminated the majority of aboveground litter inputs, soil microbial communities nonetheless continued to receive fresh organic matter inputs from three other sources: roots (including root litter and exudates), dissolved organic matter in throughfall, and labile C released from litter between monthly raking cycles. Root exudates and dissolved organic matter in throughfall in particular could provide sufficient inputs of labile C to stimulate microbial N-mining in the L− plots. Root exudates are strongly associated with priming of SOM because N-deficient plants produce exudates to stimulate microbial mineralisation of SOM, which releases plant-available N (e.g. Fontaine and Barot 2005; Fontaine et al. 2007; Bengtson et al. 2012). After root inputs, throughfall is likely to be one of the main sources of C in the L− plots, as the amounts of dissolved organic C in tropical forest throughfall are almost on a par with litter leachate from the forest floor (Schwendenmann and Veldkamp 2005). Dissolved organic matter in throughfall could stimulate the release of SOM-N in the L− plots via priming or displacement because hydrophobic compounds displace N-rich hydrophilic compounds on mineral surfaces, making mineral-bound organic N particularly vulnerable to exchange by incoming dissolved organic matter (Jilling et al. 2018).
Contrary to our second hypothesis, POM-N declined more than MAOM-N with litter removal, and the changes in the C:N ratio were not consistent across fractions, as only the C:N ratios in the largest POM and MAOM size fractions (2000–200 and 50–20 µm) increased in response to long-term litter removal. We propose that the changes in the C:N ratio of specific fractions with litter removal are likely the combined result of lower quality litter inputs and N-mining (Iversen et al. 2012). Lower quality inputs are expected in the L− plots, because nutrient-limited plants allocate more C belowground (Hessen et al. 2004) and root N concentrations declined by 10% with litter removal (Rodtassana and Tanner 2018). A greater relative contribution of root litter to SOM formation would increase the soil C:N ratio (Hatton et al. 2015) but does not explain the inconsistent increases in the C:N ratio across different size fractions. In addition, previous work at our study site revealed that litter removal has not increased root biomass or production (Sayer et al. 2006a, 2011; Rodtassana and Tanner 2018) and hence, although low-quality root litter inputs could contribute to the overall higher soil C:N ratio in the L− plots, they are unlikely to be the sole reason for higher C:N ratios of the 2000–200 and 50–20 µm fractions. However, previous decomposition studies provide evidence that reduced nutrient availability limits decomposition processes in the L− plots (Sayer et al. 2006b; Gora et al. 2018), and hence, we speculate that N-mining likely contributes to the altered C:N ratios of some SOM fractions in the L− plots.
Nitrogen-mining could explain the higher C:N ratios of the largest POM size fraction in the L− plots. Mineralisation rates of C and N in the sand fractions can be higher than in the silt or clay fractions (Turner et al. 2017) and in our study, the largest changes in C:N ratios occurred in the 2000–200 µm fraction (equivalent to the coarse sand fraction), which is generally the least stabilised and potentially the most vulnerable to degradation (Lehmann et al. 2001; Hofmockel et al. 2011; Giannetta et al. 2019). Indeed, POM-N may be the dominant source of N to microbiota (Lavallee et al. 2020) and greater fungal abundance in POM (Gude et al. 2012) could increase N mineralisation (Fontaine et al. 2011), which would explain the higher C:N ratio in the 2000–200 µm POM fraction. It is also important to note that although microbiota will use POM less efficiently than MAOM (Mooshammer et al. 2014), POM-N will be cycled more tightly with lower gaseous or leaching losses than MAOM-N (Lavallee et al. 2020), which might become increasingly important with progressive N limitation.
The higher C:N ratio of the largest MAOM size fraction (50–20 µm) in the L− plots could indicate that microbial decomposers can mine this fraction for N. MAOM is thought to be a better source of bioavailable N than POM (Fornara et al. 2011; Lavallee et al. 2020), but MAOM-N only becomes a useful source of energy and nutrients once it is destabilised from mineral protection (Lavallee et al. 2020). Nonetheless, N-mining from the 50–20 µm fraction is conceivable because the MAOM in the 50–20 µm fraction was richer in N than POM (Fig. 3b) but might not be as strongly stabilised as the MAOM in the < 20 µm size fraction (Kiem et al. 2002). Indeed, our 50–20 µm MAOM fraction corresponds to larger silt particles (Feller and Beare 1997), and the stabilisation mechanisms of SOM in the silt fraction are not well established (Paul 2016). As the C:N ratio of the < 20 µm MAOM fraction was not affected by litter removal, it is possible that the long-term reductions in plant inputs have altered the capacity of the microbiota to utilise recalcitrant substrates (Paterson et al. 2011). However, it is unclear why litter removal would result in N-mining from the 2000–200 and 50–20 µm fractions, but not the 200–50 µm fraction (Table 1; Fig. 3). It is important to note that SOM represents a continuum of progressively decomposed organic material, and separation of SOM into discrete pools will always entail limitations to interpretation (Lehmann and Kleber 2015). Although the decline in C:N ratios among fractions in the CT plots suggests that our particle size separation captured increasingly decomposed SOM pools (Poeplau et al. 2018), the observed changes in the 50–20 µm fraction with litter manipulation could indicate that this fraction contains a significant amount of small POM (Lavallee et al. 2020).
Faster SOM cycling with litter addition?
We rejected our third hypothesis of increased POM-C and N content in L+ plots, because the increases in POM-C and N with litter addition were surprisingly small and only the 200–50 µm POM fraction had higher C and N content compared to the controls (Table 1). We previously measured accelerated litter decomposition in the L+ plots (Sayer and Tanner 2010) and hence, faster cycling of organic matter (in which production rates and losses of SOM in different pools are more or less balanced) could explain why SOM C and N have not increased despite elevated inputs of plant material (Hofmockel et al. 2011; Sulman et al. 2018). In particular, gains in C and N from fresh litter inputs could be offset by priming effects, as evidenced by the disproportionate increase in soil respiration in the L+ plots (Sayer et al. 2007, 2011). We have also demonstrated reduced C storage in intermediately stabilised MAOM, which we attributed to priming by fresh organic inputs (Sayer et al. 2019). Alternatively, C-saturation of the soils at the study site might also explain the minimal gains in SOM with long-term litter addition (Lajtha et al. 2014) but based on patterns across European forests, we would expect substantial POM accumulation even in relatively C-saturated soils (Cotrufo et al. 2019). However, as tropical conditions are optimal for the decomposition of unprotected SOM (Zech et al. 1997), it may take much longer for POM to accumulate in tropical compared to temperate forests, and it is conceivable that the increased C:N ratio of the largest POM size fraction indicates the beginnings of POM accumulation in the L+ plots.
Although we measured no change in the overall C:N ratios of POM or MAOM in the L+ plots, it is surprising that the C:N ratio of the largest POM fraction (2000–200 µm) increased with litter addition. Indeed, the C:N ratio of the 2000–200 µm POM fraction in the L+ plots (c. 39) was much higher than the C:N ratio of the leaf litter (c. 28), which lends support to our interpretation that this fraction represents a source of N for microbial decomposers (Iversen et al. 2012). The higher C:N ratio of the 2000–200 µm POM fraction in the L+ plots can be largely attributed to lower N concentrations compared to the controls (Fig. 3). However, it is unclear why POM-N has declined with litter addition, as multiple lines of evidence indicate that N availability has increased in the L+ plots (Sayer et al. 2020), which should theoretically prevent microbial decomposition of SOM to acquire N (Hagedorn et al. 2003). Indeed, the high exchange rates of nitrate-N during the growing season (Fig. 6b) indicate that N supply exceeds demand in the L+ plots. Nonetheless, nutrient addition to decomposing tree boles in the L+ plots increased microbial respiration (Gora et al. 2018), which suggests that mineralization of recalcitrant substrates is nutrient-limited.
Microbial and extractable C and N
Despite substantial differences in litter inputs, the pattern of declining C:N ratios with soil particle size was similar across all treatments (Fig. 4), which is consistent with the increasing contribution of microbial decomposition products at smaller particle sizes (Lavallee et al. 2020). Across all treatments, the C:N ratios of the < 20 µm MAOM fraction (c. 11) were almost twice as high as the C:N ratios of microbial biomass (c. 6), suggesting a greater contribution of plant material to MAOM (Cotrufo et al. 2019) but neither the C:N ratio of the < 20 µm MAOM fraction, nor the C:N ratio of microbial biomass differed among treatments. The stoichiometry of microbial biomass is strongly constrained, especially compared to plant material (Cleveland and Liptzin 2007), and the smaller microbial biomass in the L− plots suggests that low bioavailability of both C and N are constraining microbial growth, which could influence the decomposition of SOM. By contrast, long-term litter removal in two temperate forests did not cause significant changes in microbial biomass (Pisani et al. 2016; Wang et al. 2017), although it is unclear whether N availability was altered by the experimental treatments at those sites. Extractable SOM had the lowest C:N ratios of all SOM fractions, reflecting its high bioavailability (Giannetta et al. 2019). The substantial decline in the C:N ratio of extractable SOM with litter addition is somewhat surprising, because it was largely due to a decline in extractable C, which was almost as large as the decline with litter removal (Fig. 5a). However, the decline in extractable C in the L+ plots likely reflects both greater incorporation of C into the microbial biomass and rapid microbial turnover of this labile C source with greater overall N availability.
Nutrient exchange rates as potential indicators of SOM mobilisation
The differences in nutrient exchange rates in the litter manipulation plots are intriguing because they potentially indicate priming of SOM with both the addition and the removal of litter, albeit via distinct pathways. Although the higher nitrate-N exchange in the L+ plots is characteristic of high N availability and a leaky N-cycle (Sayer et al. 2020), the higher exchange rates of ammonium-N in the L− plots during the peak growing season were unexpected, and could indicate increased ammonification of organic N, which lends support to our hypothesis of N-mining (Fig. 6). Increased exchange rates of ammonium-N would be expected if N-deficient plants boost root exudation to stimulate mining of N-rich compounds from SOM (Bengtson et al. 2012), and the ammonium-N is taken up by plants before it can be converted to nitrate (Schimel and Bennett 2004).
We also measured the exchange rates of Al and Fe as potential indicators of MAOM mobilisation from organo-mineral complexes. Previous work at the study site indicated that soil C priming in the L+ plots could result from the destabilisation of organo-mineral complexes involving metal oxides, whereby partially stabilised MAOM is replaced by sorptive compounds from fresh litter inputs (Sayer et al. 2019). Organic acids contained in litter leachate and root exudates can release SOM from mineral surfaces via dissolution of Fe3+ and Al3+ (Wang et al. 2014). Although we do not know the origin or species of Al and Fe captured by the resin exchange membranes, it is striking that the exchange rates of Al increased with litter removal, whereas the exchange rates of Fe increased with litter addition. As Al or Fe oxides have a high capacity to bind organic N (Jilling et al. 2018), the dissolution of organo-mineral complexes involving Al and Fe could also explain increased availability of ammonium-N and nitrate-N in the L− and L+ plots, respectively. If Al and Fe are being released from organo-mineral complexes due to active microbial priming of MAOM, or dissolution of organo-mineral complexes by organic acids in litter and root exudates (Keiluweit et al. 2015), then distinct mechanisms are at play in the L+ and L− plots, which may both result in the degradation of specific SOM C and N pools. It is important to note that differences in soil pH among litter treatments (Sayer et al. 2020) could contribute to changes in the mobility of Al and Fe. Desorption of SOM complexed with Fe oxides is hindered in low pH soils (Kleber et al. 2015), and the increase in soil pH (from 5.4–5.8) in the L+ plots could have contributed to desorption and release of Fe from MAOM. By contrast, Al becomes more mobile at low pH (Gahoonia 1993) and the decline in soil pH (from 5.4 to 5.2) with litter removal, as well as lower soil Ca (Sayer et al. 2020) and C concentrations, could enhance Al mobility in the L− plots (Dijkstra and Fitzhugh 2003). Nonetheless, as both Al and Fe play important roles in the stabilisation of SOM in tropical soils (Zech et al. 1997; Coward et al. 2017; Rasmussen et al. 2018), the links between the changes in SOM pools and the relative mobilities of ammonium-N, nitrate-N, Al or Fe in response to litter manipulation represent an intriguing avenue of research into tropical SOM storage.
Conclusions
Our study demonstrates, for the first time, concerted changes in SOM C and N in response to altered tropical forest litter inputs on a decadal time-scale. The distribution and C:N ratios of SOM in different particle size fractions reveal that distinct processes may dominate the responses to litter addition or removal. Although our study does not identify the mechanisms underpinning changes in SOM storage in response to long-term litter manipulation, our findings provide valuable evidence to inform future research into SOM stabilisation. The apparent discrepancy between the substantial declines in POM and MAOM with litter removal and the relatively minor increases with litter addition might be explained by a combination of N-mining, soil C priming and rapid SOM turnover under tropical conditions. In the L+ plots, SOM cycling was likely accelerated by increased N availability and priming of intermediately stabilised SOM by large inputs of fresh organic material. By contrast, in the L− plots, we found multiple lines of evidence for N-mining of larger SOM size fractions, whereas the consistent C:N ratio of the < 20 μm MAOM fraction across all treatments suggests that MAOM is not a substantial source of N for microbial decomposers in these soils. The increased mobilities of Fe with litter addition and Al with litter removal are particularly worthy of further attention, as they indicate changes in soil chemistry that could facilitate microbial SOM C and N priming in both treatments, albeit via distinct processes. Although we focussed on C and N as the main components of SOM, it is important to note that other nutrient elements, particularly phosphorus and sulphur, play an important role in SOM formation and stability. In-depth SOM characterisation by chemical and molecular techniques could elucidate how changes in the relative availability of C and nutrients affect organic matter storage in tropical soils.
Data availability
The data used in this study will be deposited on figshare upon acceptance.
References
Baillie I, Elsenbeer H, Barthold F et al (2007) Semi-detailed soil survey of Barro Colorado Island Panama
Bengtson P, Barker J, Grayston SJ (2012) Evidence of a strong coupling between root exudation C and N availability and stimulated SOM decomposition caused by rhizosphere priming effects. Ecol Evol 2:1843–1852
Billings SA, Ballantyne IVF (2013) How interactions between microbial resource demands soil organic matter stoichiometry and substrate reactivity determine the direction and magnitude of soil respiratory responses to warming. Glob Change Biol 19:90–102
Bimüller C, Mueller CW, von Lützow M et al (2014) Decoupled carbon and nitrogen mineralization in soil particle size fractions of a forest topsoil. Soil Biol Biochem 78:263–273
Cavelier J (1992) Fine-root biomass and soil properties in a semideciduous and a lower montane rain forest in Panama. Plant Soil 14:187–201
Chen H, Gurmesa GA, Liu L et al (2014) Effects of litter manipulation on litter decomposition in a successional gradient of tropical forests in southern China. PLoS ONE 9:e99018
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Cotrufo MF, Ranalli MG, Haddix ML et al (2019) Soil carbon storage informed by particulate and mineral-associated organic matter. Nat Geosci 12:989–994
Coward EK, Thompson AT, Plante AF (2017) Iron-mediated mineralogical control of organic matter accumulation in tropical soils. Geoderma 306:206–216
Crow SE, Lajtha K, Bowden RD et al (2009) Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. For Ecol Manag 258:2224–2232
Cusack DF, Halterman SM, Tanner EVJ et al (2018) Decadal-scale litter manipulation alters the biochemical and physical character of tropical forest soil carbon. Soil Biol Biochem 124:199–209
Dijkstra FA, Fitzhugh RD (2003) Aluminum solubility and mobility in relation to organic carbon in surface soils affected by six tree species of the northeastern United States. Geoderma 114:33–47
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol 4:216
Fahey TJ, Yavitt JB, Sherman RE et al (2011) Transport of carbon and nitrogen between litter and soil organic matter in a northern hardwood forest. Ecosystems 14:326–340
Feller C, Beare MH (1997) Physical control of soil organic matter dynamics in the tropics. Geoderma 79:69–116
Fontaine S, Barot S (2005) Size and functional diversity of microbe populations control plant persistence and long-term soil carbon accumulation. Ecol Lett 8:1075–1087
Fontaine S, Barot S, Barré P et al (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Fontaine S, Hénault C, Aamor A et al (2011) Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol Biochem 43:86–96
Fornara DA, Bardgett R, Steinbeiss S et al (2011) Plant effects on soil N mineralization are mediated by the composition of multiple soil organic fractions. Ecol Res 26:201–208
Gahoonia TS (1993) Influence of root-induced pH on the solubility of soil aluminium in the rhizosphere. Plant Soil 149:289–291
Giannetta B, Plaza C, Zaccone C et al (2019) Ecosystem type effects on the stabilization of organic matter in soils: combining size fractionation with sequential chemical extractions. Geoderma 353:423–434
Gora EM, Sayer EJ, Turner BL, Tanner EVJ (2018) Decomposition of coarse woody debris in a long-term litter manipulation experiment: a focus on nutrient availability. Funct Ecol 32:1128–1138
Grandy AS, Neff JC (2008) Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307
Grandy AS, Sinsabaugh RL, Neff JC et al (2008) Nitrogen deposition effects on soil organic matter chemistry are linked to variation in enzymes, ecosystems and size fractions. Biogeochemistry 91:37–49
Gude A, Kandeler E, Gleixner G (2012) Input related microbial carbon dynamic of soil organic matter in particle size fractions. Soil Biol Biochem 47:209–219
Hagedorn F, Spinnler D, Siegwolf R (2003) Increased N deposition retards mineralization of old soil organic matter. Soil Biol Biochem 35:1683–1692
Hatton PJ, Castanha C, Torn MS, Bird JA (2015) Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob Change Biol 21:1358–1367
Hessen DO, Ågren GI, Anderson TR et al (2004) Carbon sequestration in ecosystems: the role of stoichiometry. Ecology 85:1179–1192
Hofmockel KS, Zak DR, Moran KK, Jastrow JD (2011) Changes in forest soil organic matter pools after a decade of elevated CO2 and O3. Soil Biol Biochem 43:1518–1527
Hungate BA, Dukes JS, Shaw MR et al (2003) Nitrogen and climate change. Science 302:1512–1513
Iversen CM, Keller JK, Garten CT Jr, Norby RJ (2012) Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2-enrichment. Glob Change Biol 18:1684–1697
Jilling A, Keiluweit M, Contosta AR et al (2018) Minerals in the rhizosphere: overlooked mediators of soil nitrogen availability to plants and microbes. Biogeochemistry 139:103–122
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol Biochem 38:991–999
Kaiser M, Berher AA (2014) How does sonication affect the mineral and organic constituents of soil aggregates?: a review. J Plant Nutr Soil Sci 177:479–495
Keiluweit M, Bougoure JJ, Nico PS et al (2015) Mineral protection of soil carbon counteracted by root exudates. N Clim Change 5:588–595
Khan KS, Mack R, Castillo X et al (2016) Microbial biomass fungal and bacterial residues and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 271:115–123
Kiem R, Knicker H, Kögel-Knabner I (2002) Refractory organic carbon in particle-size fractions of arable soils I: distribution of refractory carbon between the size fractions. Org Geochem 33:1683–1697
Kirkby CA, Richardson AE, Wade LJ et al (2013) Carbon-nutrient stoichiometry to increase soil carbon sequestration. Soil Biol Biochem 60:77–86
Kleber M, Eusterhues K, Keiluweit M et al (2015) Mineral–organic associations: formation properties and relevance in soil environments. Adv Agron 130:1–140
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: tests in linear mixed effects models. J Stat Softw 82:13
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Lajtha K, Bowden RD, Crow S et al (2018) The detrital input and removal treatment (DIRT) network: insights into soil carbon stabilization. Sci Total Environ 640:1112–1120
Lajtha K, Townsend K, Kramer M et al (2014) Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 119:341–360
Lavallee JM, Soong JL, Cotrufo MF (2020) Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob Change Biol 26:261–273
Lehmann J, da Silva CM, Zech W (2001) Organic matter stabilization in a Xanthic Ferralsol of the central Amazon as affected by single trees: chemical characterization of density aggregate and particle size fractions. Geoderma 99:147–168
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528:60–68
Lopez-Sangil L, Rovira P (2013) Sequential chemical extractions of the mineral-associated soil organic matter: an integrated approach for the fractionation of organo-mineral complexes. Soil Biol Biochem 62:57–67
Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A (2014) Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Front Microbiol 5:22
Paterson E, Sim A, Osborne SM, Murray PJ (2011) Long-term exclusion of plant-inputs to soil reduces the functional capacity of microbial communities to mineralise recalcitrant root-derived carbon sources. Soil Biol Biochem 43:1873–1880
Paul EA (2016) The nature and dynamics of soil organic matter: plant inputs microbial transformations and organic matter stabilization. Soil Biol Biochem 98:109–126
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pisani O, Lin LH, Lun OO et al (2016) Long-term doubling of litter inputs accelerates soil organic matter degradation and reduces soil carbon stocks. Biogeochemistry 127:1–14
Poeplau C, Don A (2014) Effect of ultrasonic power on soil organic carbon fractions. J Plant Nutr Soil Sci 177:137–140
Poeplau C, Don A, Six J, Kaiser M et al (2018) Isolating organic carbon fractions with varying turnover rates in temperate agricultural soil: a comprehensive method comparison. Soil Biol Biochem 125:10–26
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rasmussen C, Heckman K, Wieder WR et al (2018) Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137:297–306
Rodtassana C, Tanner EVJ (2018) Litter removal in a tropical rain forest reduces fine root biomass and production but litter addition has few effects. Ecology 99:735–742
Sayer EJ, Tanner EVJ (2010) Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. J Ecol 98:1052–1062
Sayer EJ, Tanner EVT, Wright SJ et al (2012) Comparative assessment of lowland tropical forest nutrient status in response to fertilization and litter manipulation. Ecosystems 15:387–400
Sayer EJ, Heard MS, Grant HK et al (2011) Soil carbon release enhanced by increased tropical forest litterfall. N Clim Change 1:304–307
Sayer EJ, Tanner EVJ, Cheesman AW (2006a) Increased litterfall changes fine root distribution in a moist tropical forest. Plant Soil 281:5–13
Sayer EJ, Tanner EVJ, Lacey AL (2006b) Litter quantity affects early-stage decomposition and meso-arthropod abundance in a moist tropical forest. For Ecol Manag 229:285–293
Sayer EJ, Powers JS, Tanner EVJ (2007) Increased litterfall in tropical forests boosts the transfer of soil CO2 to the atmosphere. PLoS ONE 2:e1299
Sayer EJ, Rodtassana C, Sheldrake M et al (2020) Revisiting nutrient cycling by litterfall: insights from 15 years of litter manipulation in old-growth lowland tropical forest. Adv Ecol Res 62:173–223
Sayer EJ, Lopez-Sangil L, Crawford JA et al (2019) Tropical forest soil carbon stocks do not increase despite 15 years of doubled litter inputs. Sci Rep 9:18030
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Schwendenmann L, Veldkamp E (2005) The role of dissolved organic carbon dissolved organic nitrogen and dissolved inorganic nitrogen in a tropical wet forest ecosystem. Ecosystems 8:339–351
Signorell A, Aho K, Anderegg N et al (2018) DescTools: tools for descriptive statistics. R package version 0.99 24
Stockmann U, Adams MA, Crawford JW et al (2013) The knowns known unknowns and unknowns of sequestration of soil organic carbon. Agri Ecosyst Environ 164:80–99
Sulman BN, Moore JA, Abramoff R et al (2018) Multiple models and experiments underscore large uncertainty in soil carbon dynamics. Biogeochemistry 141:109–123
Sulzman EW, Brant JB, Bowden RD, Lajtha K (2005) Contribution of aboveground litter belowground litter and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 73:231–256
Tanner EVJ, Sheldrake MW, Turner BL (2016) Changes in soil carbon and nutrients following 6 years of litter removal and addition in a tropical semi-evergreen rain forest. Biogeosciences 13:6183
Turner S, Meyer-Stüve S, Schippers A et al (2017) Microbial utilization of mineral-associated nitrogen in soils. Soil Biol Biochem 104:185–196
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vitousek PM (1984) Litterfall nutrient cycling and nutrient limitation in tropical forests. Ecology 65:285–298
Wang Z, Xu Z, Zhao J et al (2014) Effects of low-molecular weight organic acids on soil micropores and implication for organic contaminant availability. Commun Soil Sci Plant Anal 45:1120–1132
Wang J, Pisani O, Lin LH et al (2017) Long-term litter manipulation alters soil organic matter turnover in a temperate deciduous forest. Sci Total Environ 607:865–875
Zech W, Senesi N, Guggenberger G et al (1997) Factors controlling humification and mineralization of soil organic matter in the tropics. Geoderma 79:117–161
Acknowledgements
We thank F. Valdez for field assistance, S.J. Wright, E.V.J. Tanner and the Smithsonian Tropical Research Institute for hosting the fieldwork, and A. Ryan for her excellent lab management. This work was funded by a European Research Council Starting Grant, awarded to E.J.S. under the European Union's Seventh Framework Programme (FP/2007-2013), ERC Grant Agreement No. 307888.
Author information
Authors and Affiliations
Contributions
EJS conceived the study, analysed the data, and wrote the manuscript; LLS designed and performed the laboratory work with CB and AJB; LMB and DKB performed sampling and measurements in the field with BC, CR provided additional data, and all authors contributed to the presentation and interpretation of results. Co-authors are listed in alphabetical order.
Corresponding author
Additional information
Responsible Editor: Samantha R. Weintraub.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sayer, E.J., Baxendale, C., Birkett, A.J. et al. Altered litter inputs modify carbon and nitrogen storage in soil organic matter in a lowland tropical forest. Biogeochemistry 156, 115–130 (2021). https://doi.org/10.1007/s10533-020-00747-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00747-7