Abstract
SiC focus rings are mount on the stage at the surround of wafer in the reactive ion etching chamber, in order to make the etching rate uniform. SiC is used for the material of focus ring because it has higher plasma resistance than Si. We investigated a new method to recycle SiC without using the chemical vapor deposition (CVD) method. The recycling process consists of grinding, dry cleaning, hot isostatic pressing (HIP), atmospheric burning, and aqueous solution cleaning. As a result, diffusion bonding with a tensile strength of 100 kg cm−2 or more was realized by reducing the surface roughness at the diffusion bonding surface to 0.1 μm or less. It was confirmed that the SiC surface was oxidized with thickness 135 nm by atmospheric burning in air ambience. It was also confirmed that the oxide layer at the SiC surface was completely removed by the aqueous solution cleaning. We have reduced the cost to 50% for SiC focus ring replacement by using the recycle method.
Export citation and abstract BibTeX RIS
Since the number of stacked layers has increased with increase in capacity of flash memories, the manufacturing process step increases. In particular, reactive ion etching (RIE) requires capability for high aspect ratio. A high-frequency voltage is applied to generate plasma discharge, and fine processing is performed to the wafer surface using sputtering by ions and/or chemical reaction by radicals. 1 Focus rings are put at the surround the wafer in the RIE chamber, in order to make the etching rate uniform. 2,3 Si focus ring is conventionally used, and is etched together with wafer surface. 4 Therefore, the etching rate around the wafer peripheral changes. Therefore, SiC is used for the material of focus ring because it has higher plasma resistance than Si. 5 However, even if the focus ring is made by SiC and its lifetime is for several hundred hours, the etching rate at the outer periphery of the wafer will fluctuate. Therefore, it is needed to replace the SiC focus ring itself.
SiC is fabricated by sublimation method, reaction sintering method, recombination method, hot pressing method, and chemical vapor deposition (CVD). 6–9 The SiC material used for the focus ring is required to have high strength and high purity. The CVD-SiC process is more complicated than CVD-Si process, and the film deposition rate is as low as several μm h−1. 10 And also, CVD-SiC process uses a large amount of electric power, resulting in large environmental impact. In addition, reaction by-products in the exhaust gas are regulated from the aspect of pollution, and exhaust gas treatment is required for equipment.
In this study, we investigated a new method to recycle SiC without using CVD method. Consumed SiC focus rings were recycled by diffusion bonding technique with hot isostatic pressing (HIP). There is also a method in which a metal is inserted between the bonding surfaces for the purpose of lowering and promoting the bonding temperature. 11–13 However, since the highly pure SiC is needed, we have studied a bonding method that does not use metal insertion. 8,14
Experimental
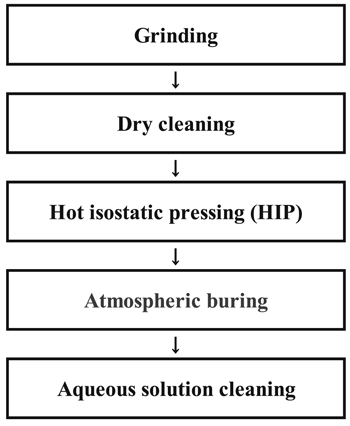
Figure 1 shows a recycle process flow of the SiC focus ring. The recycle process flow consists of grinding, dry cleaning, HIP, atmosphere burning, and aqueous solution cleaning. Table I summarizes conditions of recycle processes. Grinding is performed to pave the shape of the focus ring consumed by the plasma process, and to reduce the surface roughness of the SiC focus ring surface for bonding. Next, the metal adsorbed during the grinding and the bonding side surface were cleaned by dry cleaning at 1000 °C under HCl atmosphere. After dry cleaning, HIP was carried out to bond the bonding surfaces of two SiC focus ring together. HIP method is used for surface bonding by applying pressure at a high temperature and in high-pressure atmosphere. Next, the SiC surface is oxidized in air by burning, and then the oxide layer at the SiC surface is removed by aqueous solution cleaning. Fluorocarbon and the metal adhered by the grinding process cannot be removed by dry cleaning and remain in the unpolished part of SiC surface. The surface of SiC is oxidized by atmospheric burning, and the oxide layer on the surface of SiC is removed together with the fluorocarbon and the metal in the oxide layer by cleaning with HF based aqueous solution. Aqueous solution cleaning was performed in Kurifresh T-120 by Kuritec Service Co., Ltd.
Figure 1. Recycle process flow for SiC focus ring.
Download figure:
Standard image High-resolution imageTable I. Conditions of recycle processes
| Grinding | Dry Cleaning | HIP | Atomspheric buring | Aqueous solution cleaning | |
|---|---|---|---|---|---|
| Wheel rotation speed (rpm) | 300 | ||||
| Work rotation speed (rpm) | 60 | ||||
| Feed speed (μm min−1) | 20 | ||||
| Pressure (MPa) | 1.96 | 191 | |||
| Process Time (hour) | 2 | 1 | 8 | 3 | |
| Temperature (°C) | 1000 | 1980 | 1000 | 25 | |
| Gas | HCl | Ar | Air | ||
| Flow Rate (ℓ min−1) | 2 |
To confirm whether this SiC recycle method is effective, we conducted an experiment using samples with the size of height 20 mm × width 10 mm × distance 1.5 mm. After HIP, the distance became 3.0 mm. In addition, it has been reported in previous research that the adhesion strength of the diffusion bonding interface changes depending on the surface roughness. 15 Therefore, the surface roughness should be reduced from 0.4 to 0.05 μm for diffusion bonding.
Since SiC has various crystal structures such as a cubic structure (3C), a hexagonal structure (4H, 6H), and a rhombohedral structure (15R), the crystal structure may change by heating in HIP processing. Therefore, the crystal structures before and after HIP were analyzed by X-ray diffraction (XRD) method (Bruker, D8DISCOVER).
The adhesion strength was measured using an ultrasonic test (UT) (FineSAT FS300Ⅲ, Hitachi High-Technologies) to confirm the SiC bonding strength, and a bond tester (BT) (Dage 4000, Norton Advanced Technology). The reason to use UT technique is that the strength should be measured nondestructively. Also, BT can easily measure the bonding strength without using an adhesive promoter. Therefore, the relationship between the reflection intensity by UT and the tensile strength by BT was examined. UT conditions are as follows; probe frequency 15 MHz, depth of focus 50 mm, scan pitch 0.5 μm. Also, BT conditions are test speed 200 μm s−1, maximum force 100 kg cm−2. A scanning transmission electron microscope (STEM) (HD-2700, Hitachi High-Technologies) was used for cross-sectional observation for the bonding interface. In order to confirm the existence of oxide layer at the SiC surface after atmosphere burning in air, X-ray photoelectron spectroscopy (XPS) measurement (Versa Probe II, ULVAC-PHI) was carried out. The measurement was performed with an X-ray source of Al-kα radiation and a photoelectron detection angle of 90°. Depth profile analysis was performed by XPS and Ar sputtering to confirm the thickness of SiO2 on SiC surface due to burning in air. Ar sputter rate is 5.88 nm min−1. An interface of the oxide layer on the SiC surface and base material of SiC was set to a depth where the O1s peak in the binding energy of 533 eV was 50%. The reason for setting to 50% is that the interface between the oxide layer on the SiC surface and the base material is not clearly exposed due to the influence of the surface roughness of the SiC surface. Also, in order to confirm whether the oxide layer on the SiC surface could be removed by aqueous solution cleaning, the SiC surface before and after aqueous solution cleaning was measured using XPS (Versa Probe II, ULVAC-PHI).
Results and Discussion
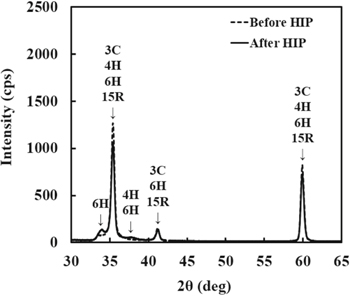
Figure 2 shows XRD spectra before and after HIP process. The stronger peaks at angles (2θ) of 35.4°, 41.3° and 59.9° are 3C-SiC structure. After HIP process, the peak intensity at angles (2θ) of 34.1° and 38.0° slightly increased. Yasuda has previously reported that heating 3C-SiC above 2000 °C the crystal structure partly changed to 6H-SiC structure. 16 Therefore, it is possible that crystal structure partly changed to 6H-SiC due to temperature variations during HIP processing.
Figure 2. XRD spectra before and after HIP process.
Download figure:
Standard image High-resolution imageFigure 3 shows the relationship between the SiC bonding surface roughness and the UT reflection intensity. As the SiC bonding surface roughness decreased, the UT reflection intensity decreases. The UT reflection intensity increases due to the inner defects such as a void. Therefore, defect less bonding can be performed when the SiC bonding surface roughness is small. The UT reflection intensity was constant under the surface roughness of 0.1 μm.
Figure 3. Relationship between bonding surface roughness and UT reflection intensity.
Download figure:
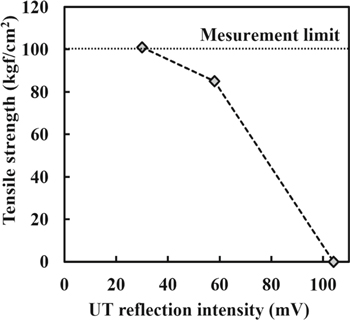
Standard image High-resolution imageFigure 4 shows the relationship between the tensile strength and the UT reflection intensity. The tensile strength increases as the UT reflection intensity decreases. These results show, when the surface roughness is low, diffusion bonding is promoted. This is because that internal defects such as voids are reduced and tensile strength is increased. Ohashi et al. have reported that tensile strength increased as surface roughness decreased, and our results are consistent with this report. 15 From the above results, the tensile strength increases with decreasing the surface roughness. These results indicate that the reduction of surface roughness promotes diffusion bonding due to increase in the contact area of the SiC bonding surface.
Figure 4. Relationship between tensile strength and UT reflection intensity.
Download figure:
Standard image High-resolution imageFigures 5a–5c show cross-sectional images for an optical microscope, SEM, STEM, respectively. The observation sample surface was grinded to reduce the SiC bonding surface roughness to 0.05 μm. In the cross-section of the optical microscope image, the diffusion bonded part and void were observed. The black dots at the bonding interface are voids. A previous study by Ohashi reported that Ar remains in the voids and prevents shrinkage of the voids when diffusion bonding is performed in an Ar atmosphere. 17 Since HIP was processed in Ar atmosphere, it is assumed that Ar remained in the voids on the SiC bonding surface. This result is consistent with the previous report by Ohashi. Therefore, we observed the cross-sections of diffusion bonding interface by SEM and STEM. In the SEM and STEM images, diffusion bonding of SiC was confirmed at the bonding interface. Also, void is clearly observed in the SEM image at the bonding interface and the voids are brightly observed in the STEM image. These results indicate that diffusion bonding is performed when the SiC bonding surface roughness is set to 0.05 μm.
Figure 5. (a) Optical microscope, (b) SEM cross-sectional and (c) STEM cross-sectional images of SiC bonding interface, where SiC bonding surface roughness is 0.05 μm.
Download figure:
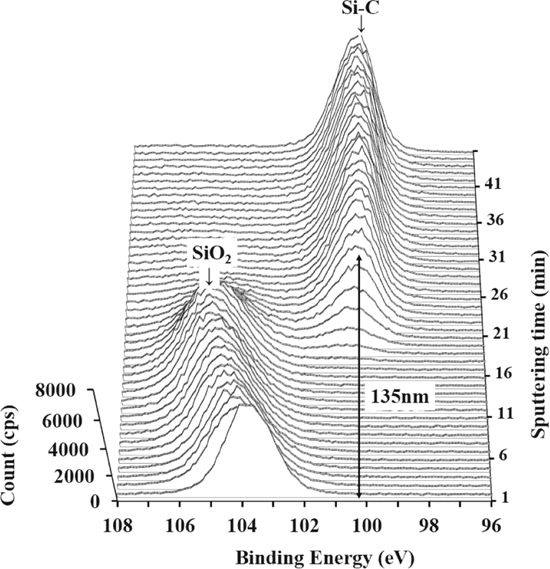
Standard image High-resolution imageFigure 6 shows Si2p narrow scan spectra by XPS depth analysis. The SiO2 peak at a binding energy of 104.0 eV indicates an oxide layer at the SiC surface, and the Si–C peak at a binding energy of 100.1 eV indicates the base material of SiC. At the beginning of sputtering, the SiO2 peak was high, and then it decreased. As sputtering progressed, the Si–C peak became stronger. This shows that the oxide layer at the SiC surface was removed by Ar sputter and the SiC base material appeared. From this result, it was confirmed that the SiC surface was oxidized with thickness 135 nm.
Figure 6. Si2p narrow scan spectra by XPS depth analysis.
Download figure:
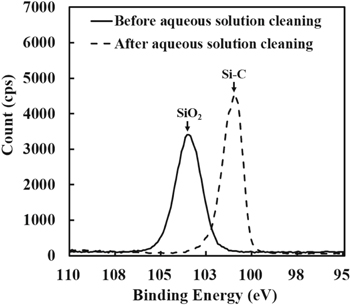
Standard image High-resolution imageFigure 7 shows surface analysis results by XPS before and after aqueous solution cleaning. Before the aqueous solution cleaning, the surface was oxidized as mentioned above. Therefore, a strong SiO2 peak was observed at the binding energy of 104 eV. However, after the aqueous solution cleaning, the Si-C peak at the binding energy of 100.1 eV became strong. This result is consistent with the results obtained by XPS depth analysis. Therefore, it was confirmed that the oxide layer at the SiC surface was completely removed by the aqueous solution cleaning.
Figure 7. Surface analysis results by XPS before and after cleaning with aqueous solution.
Download figure:
Standard image High-resolution imageConclusions
We verified SiC focus ring recycle method by diffusion bonding using HIP. As the recycle procedure, it was confirmed that the SiC focus ring can be recycled by series processes of grinding, dry cleaning, HIP, atmosphere burning, and aqueous solution cleaning. After HIP process, the bonding interface of SiC can make strong by setting the roughness of bonding interface under 0.1 μm. It was found that aqueous solution cleaning after atmosphere burning can remove oxide layer at the SiC surface. We have reduced the cost to 50% for SiC focus ring replacement by using the recycle method. In the future, HIP method will be used to recycle of SiC focus ring and contribute to waste reduction.