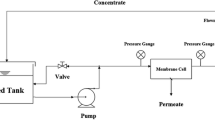
Abstract
Poly(vinylidene fluoride) (PVDF) was incorporated in Cellulose acetate (CA) to prepare polymeric blend membrane to enhance the antifouling properties and rejection. Blend membranes consist of different concentrations (0, 2.5 and 5.0 wt%) of polyvinylpyrrolidone (PVP), which was the hydrophilic polymer additive and pore forming agent. The existence of membrane functional groups was analyzed by ATR-FTIR spectroscopy. AFM and SEM were conducted to explain the surface morphology of the synthesized blend membranes. Membrane properties were examined by contact angle, porosity and equilibrium water content (EWC). The membranes thermal and mechanical properties were determined by performing TGA and tensile test. Membrane performance was assessed by pure water flux (PWF), rejection, antifouling properties using bovine serum albumin (BSA) and Sodium alginate (SA) solution. In this study, CPA-2 membrane showed high PWF of 269.82 L m−2 h−1, flux recovery ratio (FRR) against BSA and SA was 94% and 92%, respectively. Rejection of BSA and SA was found to be 83% and 86%, respectively. From the results, it was significant that the hydrophilic additive PVP blended membrane ameliorated and showed better results in surface roughness, hydrophilicity, thermal and mechanical stability. Hence, CPA-2 membrane would exhibit less susceptibility to fouling with enhanced PWF, permeability and selectivity.
Similar content being viewed by others
Abbreviations
- A:
-
area of the membrane [cm2]
- BSA:
-
bovine serum albumin
- CA:
-
cellulose acetate
- Cf :
-
concentration of the feed [gL−1]
- C p :
-
concentration of the permeate [gL−1]
- CPA-0:
-
CA/PVDF without PVP (0 wt%)
- CPA-1:
-
CA/PVDF with PVP (2.5 wt%)
- CPA-2:
-
CA/PVDF with PVP (5 wt%)
- DMF:
-
dimethyl formamide
- EWC:
-
equilibrium water content
- FRR:
-
flux recovery ratio
- IDT:
-
intial decomposition temperature [oC]
- Jp :
-
protein permeate flux [L m−2 h−1]
- Js :
-
sodium alginate permeate flux [L m−2 h−1]
- Jv1 :
-
initial pure water flux [L m−2 h−1]
- Jv2 :
-
final pure water flux [L m−2 h−1]
- L:
-
thickness of the membrane [cm]
- PB:
-
phosphate buffer
- PVDF:
-
poly(vinylidene fluoride)
- PVP:
-
polyvinylpyrrolidone
- PWF:
-
pure water flux
- Q:
-
volume of the permeate per unit time [m−3s−1]
- Ra :
-
mean roughness profile [nm]
- Rir :
-
irreversible protein fouling
- rm :
-
average pore size [nm]
- Rmax :
-
maximum height of the roughness profile [nm]
- Rq :
-
root mean square deviation roughness [nm]
- Rr :
-
reversible protein fouling
- Rt :
-
total protein fouling
- RT :
-
total height of the roughness profile [nm]
- SA:
-
sodium alginate
- Sc :
-
spreading coefficient [mNm−1]
- Td :
-
thermal degradation at 5% wt loss [oC]
- TMP:
-
trans membrane pressure [MPa]
- Wa :
-
work of adhesion [mNm−1]
- WCA:
-
water contact angle
- Wd :
-
weight of the dry sample [g]
- Ww :
-
weight of the wet sample [g]
- β :
-
constant value of 0.0001024 mJm−2
- ε :
-
porosity of the membrane
- η :
-
waterviscosity[Pa s]
- θ :
-
solid-liquid contact angle
- ρ :
-
density of pure water [g cm−3]
- ϒtv :
-
interfacial free energies of liquid-vapor [mNm−1]
- ϒst :
-
interfacial free energies of solid-liquid [mNm−1]
- ϒsv :
-
interfacial free energies of solid-vapor [mNm−1]
- ΔP:
-
trans-membrane pressure [Pa]
- Δt:
-
time duration of permeate [h]
References
S. Rajesh, K. H. Shobana, S. Anitharaj and D. R. Mohan, Ind. Eng. Chem. Res., 50, 5550 (2011).
M. Sivakumar, D. R. Mohan and R. Rangarajan, J. Membr. Sci., 268, 208 (2006).
A. W. Zularisam, A. F. Ismail, M. R. Salim, M. Sakinaha and H. Ozaki, Desalination, 212, 191 (2007).
M. Hashino, T. Katagiri, N. Kubota, Y. Ohmukai, T. Maruyama and H. Matsuyama, J. Membr. Sci., 366, 258 (2011).
G. F. Crozes, J. G. Jacangelo, C. Anselme and J. M. Laine, J. Membr. Sci., 124, 63 (1997).
W. Yuan and A. L. Zydney, Environ. Sci. Technol., 34, 5043 (2000).
M. Liu, C. Xiao and X. Hu, Desalination, 298, 59 (2012).
D.-Q. Cao, X.-D. Hao, Z. Wang, X. Song, E. Iritani and N. Katagiri, J. Membr. Sci., 535, 312 (2017).
K. Katsoufidou, S. G. Yiantsios and A. J. Karabelas, J. Membr. Sci., 300, 137 (2007).
K. Kimura, Y. Hane, Y. Watanabe, G. Amy and N. Ohkuma, Water Res., 38, 3431 (2004).
M. H. Razzaghi, A. Safekordi, M. Tavakolmoghadam, F. Rekabdar and M. Hemmati, J. Membr. Sci., 470, 547 (2014).
B. Han, D. Zhang, Z. Shao, L. Kong and S. Lv, Desalination, 311, 80 (2013).
H. Kamal, F. M. Abd-Elrahim and S. Lofty, J. Radiat. Res. Appl. Sci., 7, 146 (2014).
S. Vidya and D. Mohan, Sep. Sci. Technol., 45, 740 (2010).
Y. Ertas and T. Uyar, Crabohyd. Polym., 177, 378 (2017).
M. K. Irfana, Arun M. Isloor, A. F. Ismail, O. Abdulrahman and H. K. Fun, Desalin. Water Treat., 57, 19810 (2015).
L.-F. Fang, H.-Y. Yang, L. Cheng, N. Kato, S. Jeon, R. Takagi and H. Matsuyama, Ind. Eng. Chem. Res., 56, 11302 (2017).
E. Eren, A. Sarihan, B. Eren, H. Gumus and F. O. Kocak, J. Membr. Sci., 475, 1 (2015).
F. S. Dehkordi, M. Pakizeh and M. Namvar-Mahboub, Appl. Clay Sci., 105, 178 (2015).
K. A. Gebru and C. Das, J. Environ. Manage., 200, 283 (2017).
M. S. Muhamad, M. R. Salim and W.-J. Lau, Korean J. Chem. Eng., 32, 2319 (2015).
S. Abdolhamid, M. Toraj, R. M. Behbahani and M. Hemati, Adv. Polym. Technol., 34, 21494 (2015).
K. A. Gebru and C. Das, Chin. J. Chem. Eng., 25, 911 (2017).
J. S. Beril Melbiah, D. Nithya and D. Mohan, Colloids Surf. A Physicochem. Eng. Asp., 516, 147 (2017).
Pramila, J. S. Beril Melbiah, D. Rana, N. Nagendra Gandhi, A. Nagendran and D. Mohan, Polym. Test., 67, 218 (2018).
D. Y. Kwok and A. W. Neumann, Colloids Surf. A: Physicochem. Eng. Asp., 161, 31 (2000).
S. Zha, J. Yu, G. Zhang, N. Liu and R. Lee, RSC Adv., 5, 105692 (2015).
M. Safarpour, V. Vatanpour and A. Khataee, Desalination, 393, 65 (2016).
R. S. Hebbar, A. M. Isloor, A. F. Ismail, S. J. Shilton, O. Abdulrahman and H. K. Fun, New J. Chem., 39, 6141 (2015).
P. Maheswari, P. Barghava and D. Mohan, J. Polym. Res., 20, 1 (2013).
J. Dasgupta, S. Chakraborty, J. Sikder, R. Kumar, D. Pal, S. Curcio and E. Drioli, Sep. Purif. Technol., 133, 55 (2013).
M. Kumar and J. Lawler, Sep. Purif. Technol., 130, 112 (2014).
W.-Z. Lang, L.-F. Chu and Y.-J. Guo, J. Appl. Polym. Sci., 121, 1961 (2011).
R. S. Hebbar, A. M. Isloor, B. Prabhu, Inamuddin, A. M. Asiri and A. F. Ismail, Sci. Rep., 8, 4665 (2018).
G. Zhang, L. Zhang, S. Lu, Q. Meng, C. Shen and J. Zhang, J. Membr. Sci., 436, 163 (2013).
A. K. Nair, A. M. Isloor, R. Kumar and A. F. Ismail, Desalination, 322, 69 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nainar, M.G., Jayaraman, K., Meyyappan, H.K. et al. Antifouling properties of poly(vinylidene fluoride)-incorporated cellulose acetate composite ultrafiltration membranes. Korean J. Chem. Eng. 37, 2248–2261 (2020). https://doi.org/10.1007/s11814-020-0653-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-020-0653-8