Abstract
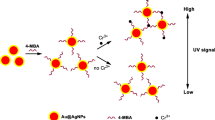
In this study, a selective and easy colorimetric probe was developed for the detection of silver ions. This system relied on the aggregation of gold nanoparticles (AuNPs) with ammonium pyrrolidine dithiocarbamate (APDC) and its anti-aggregation in the presence of silver ions. APDC caused the aggregation of AuNPs which resulted in color change from red to blue. However, in the presence of silver ions, because of their ability to complex with APDC, the aggregation of AuNPs was inhibited and color change occurred in the opposite direction by increasing Ag+ concentration (blue to red). Under optimum conditions, this system exhibited high selectivity to Ag+ over other tested metal ions. This novel probe had advantages such as wide linear range (0.05–0.9 µM) and low detection limit (20 nM). The recovery analysis of river water as real sample confirmed that the proposed colorimetric sensor could be successfully employed in the determination of Ag+ in real water samples.







Similar content being viewed by others
REFERENCES
Bhardwaj, V.K., Singh, N., Hundal, M.S., and Hundal, G., Tetrahedron, 2006, vol. 62, no. 33, p. 7878.
Yeh, Y.-C., Creran, B., and Rotello, V.M., Nanoscale, 2012, vol. 4, no. 6, p. 1871.
Du, J., Zhu, B., Peng, X., and Chen, X., Small, 2014, vol. 10, no. 17, p. 3461.
Duan, J., Yin, H., Wei, R., and Wang, W., Biosens. Bioelectron., 2014, vol. 57, p. 139.
Lin, C.-Y., Yu, C.-J., Lin, Y.-H., and Tseng, W.-L., Anal. Chem., 2010, vol. 82, no.16, p. 6830.
Yang, C.-G., Zhang, M., and Xu, Z.-R., Anal. Methods, 2015, vol. 7, no. 3, p. 1110.
Li, B., Du, Y., and Dong, S., Anal. Chim. Acta, 2009, vol. 644, nos. 1–2, p. 78.
Zhan, S., Wu, Y., He, L., Wang, F., Zhan, X., Zhou, P., and Qiu, S., Anal. Methods, 2012, vol. 4, no. 12, p. 3997.
Zhan, S., Xu, H., Zhan, X., Wu, Y., Wang, L., Lv, J., and Zhou, P., Microchim. Acta, 2015, vol. 182, nos. 7–8, p. 1411.
Zhang, Y., Liu, W., Zhang, W., Yu, S., Yue, X., Zhu, W., Zhang, D., Wang, Y., and Wang, J., Biosens. Bioelectron., 2015, vol. 72, p. 218.
Pu, W., Zhao, Z., Wu, L., Liu, Y., and Zhao, H., J. Nanosci. Nanotechnol., 2015, vol. 15, no. 8, p. 5524.
Zhu, G. and Zhang, C., Analyst, 2014, vol. 139, no. 24, p. 6326.
Safavi, A., Ahmadi, R., and Mohammadpour, Z., Sens. Actuators, B, 2017, vol. 242, p. 609.
Frens, G., Nature, 1973, vol. 241, no. 1, p. 20.
Shang, J. and Gao, X., Chem. Soc. Rev., 2014, vol. 43, no. 21, p. 7267.
Kazi, T.G., Afridi, H.I., Shah, F., Arain, S.S., Brahman, K.D., Ali, J., and Arain, M.S., Arab. J. Chem., 2016, vol. 9, no. 1, p. 105.
Wang, L., Rangger, G.M., Ma, Z., Li, Q., Shuai, Z., Zojer, E., and Heimel, G., Phys. Chem. Chem. Phys., 2010, vol. 12, no. 17, p. 4287.
Daniel, M.-C. and Astruc, D., Chem. Rev., 2004, vol. 104, no. 1, p. 293.
Liu, D., Qu, W., Chen, W., Zhang, W., Wang, Z., and Jiang, X., Anal. Chem., 2010, vol. 82, no. 23, p. 9606.
Chen, L., Li, J., and Chen, L., ACS Appl. Mater. Interfaces, 2014, vol. 6, no. 18, p. 15897.
Chen, X., Zu, Y., Xie, H., Kemas, A.M., and Gao, Z., Analyst, 2011, vol. 136, no. 8, p. 1690.
Xu, X., Qiao, J., Qi, L., Wang, L., and Zhang, S., Sci. China Chem., 2015, vol. 58, no. 6, p. 1065.
Feng, D.-Q., Liu, G., Zheng, W., Liu, J., Chen, T., and Li, D., Chem. Commun., 2011, vol. 47, no. 30, p. 8557.
Sung, Y.-M. and Wu, S.-P., Sens. Actuators, B, 2014, vol. 197, p. 172.
He, Y., Liang, Y., and Song, H., Plasmonics, 2016, vol. 11, no. 2, p. 587.
Lou, T., Chen, Z., Wang, Y., and Chen, L., ACS Appl. Mater. Interfaces, 2011, vol. 3, no. 5, p. 1568.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Behrooz Azizi, Farhadi, K. & Samadi, N. Lable-Free Gold Nanoparticles in the Presence of Ammonium Pyrrolidine Dithiocarbamate as a Selective and Sensitive Silver Ion Colorimetric Probe. J Anal Chem 75, 1546–1553 (2020). https://doi.org/10.1134/S1061934820120035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061934820120035