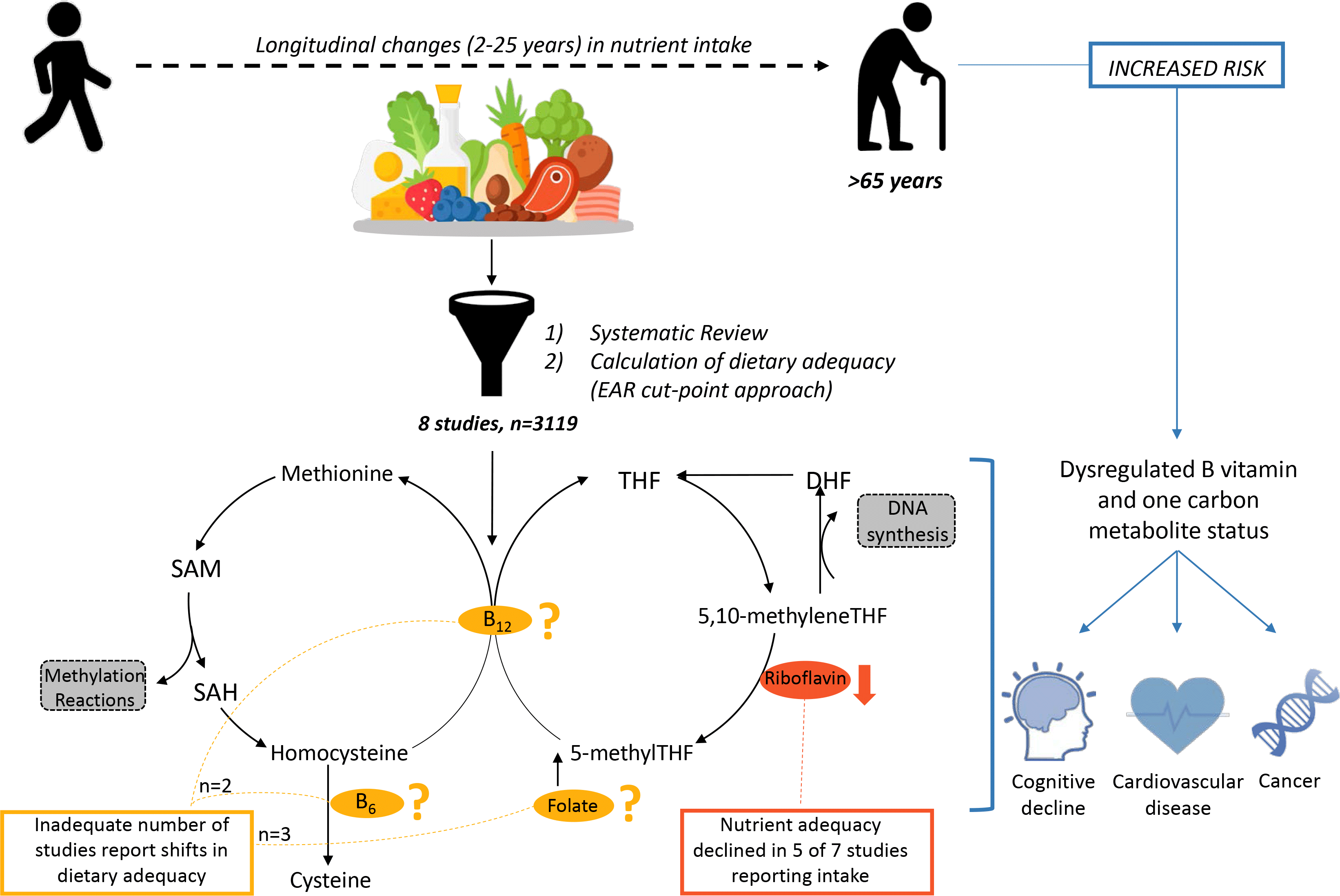
Population ageing is occurring rapidly, with estimates that the global proportion of adults aged over 65 years will increase to 16 % by 2050(1). This profound demographic shift has important individual and societal consequences due to the increased chronic disease risk associated with age(Reference Porter, Hoey and Hughes2,3) . Maintaining optimal nutrition status is a key modifiable factor that contributes to successful ageing and hence is increasingly important for older adults(Reference Troesch, Hoeft and McBurney4–Reference Bruins, Van Dael and Eggersdorfer6). Although older adults are at greater risk of nutritional deficiencies(Reference Morley7,Reference Morley8) , changes in micronutrient intake specific to ageing have not yet been systematically reported.
Among essential nutrients, dietary adequacy of B vitamins involved in the regulation of one-carbon metabolism (folate, riboflavin, vitamins B6 and B12) is of particular interest in older adults given their co-regulation of homocysteine, DNA synthesis and methylation reactions(Reference Selhub9). Accordingly, B vitamins and homocysteine, as a marker of one-carbon metabolism, are implicated in a number of common diseases of ageing, including vascular disease(Reference Wald, Morris and Wald10,11) , cancers(Reference Ericson, Sonestedt and Gullberg12,Reference Giovannucci13) , cognitive impairment(Reference Quadri, Fragiacomo and Pezzati14–Reference Wang, Wahlin and Basun16) and osteoporosis(Reference Sato, Honda and Iwamoto17,Reference McLean, Jacques and Selhub18) . Research on the association between B vitamins, homocysteine and health outcomes has typically focused on understanding the involvement of folate, and to a lesser extent vitamin B12 (Reference Araújo, Martel and Borges19–Reference Herrmann, Herrmann and Obeid24). However, folate metabolism is closely interlinked with these other vitamins involved in one-carbon metabolism, and inadequate status of any one of the four nutrients can perturb the complex pathways comprising one-carbon metabolism(Reference Porter, Hoey and Hughes2).
Despite the importance of adequate nutritional intake, this becomes increasingly difficult to achieve for older adults owing to a complex interaction of physiological, psychological and biological factors. Older adults experience sensory changes, such as diminished taste and smell, and neuroendocrine changes that affect appetite and satiety, and face challenges with swallowing, chewing and limited mobility. Together with socio-economic and psychological changes that are often concurrent with advancing age, older adults are particularly vulnerable to inadequate micronutrient intake and status(Reference Morley7,Reference Morley8) . Indeed, a systematic review by ter Borg et al. (Reference ter Borg, Verlaan and Hemsworth25) reported a high prevalence of inadequate dietary intake, upwards of 25–40 %, of folate and related vitamins in older adults, including riboflavin, folate and vitamin B6. However, these findings, echoed by others, are generally limited to cross-sectional designs or cross-age comparisons(Reference Olsen, Halkjaer and van Gils26–Reference Volkert, Kreuel and Heseker31). Although these cross-sectional findings bolster the widespread notion that intake of B vitamins and other nutrients declines with age, they fail to describe how nutrient intake changes due to the ageing process.
Despite targeted efforts to better understand the intake of this group of B vitamins(Reference McNulty and Scott32–Reference Tucker, Raats, de Groot and van Staveren34), it remains unclear how dietary intake of these nutrients changes during ageing, and how this impacts health outcomes. Accordingly, to better understand how to optimise the nutritional status of B vitamins, the nature of age-related changes in dietary intake must first be characterised. The current systematic review therefore aims to explore how the prevalence of dietary adequacy of folate, riboflavin and vitamins B6 and B12 alters with age in community-dwelling older adults.
Methods
The present systematic review followed the reporting checklist as part of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement(Reference Moher, Shamseer and Clarke35). The protocol of the current review was registered at PROSPERO (CRD42018104364), an international database of prospectively registered systematic reviews in health and social care. The Population, Intervention, Comparison, Outcomes and Study design criteria used to define our research question are summarised in Table 1.
Table 1. PICOS criteria employed to define our research question

PICOS, Population, Intervention, Comparison, Outcomes and Study.
Search strategy
The electronic databases BIOSIS, CINAHL, Embase and MEDLINE were searched for publications between 1990 and 8 March 2018. Medical Subject Headings, Medical Subject Headings major topics and free text terms were developed under four group headings (B-group vitamins, dietary intake, older adults and study design) for Embase, MEDLINE and Biosis databases, which were modified for the CINAHL search preferences. Medical Subject Headings terms were applied to subject headings where possible, and keywords were searched for in ‘many places’ or in a similar search field. All subject headings were joined with AND to develop the search string. An example of the full search strategy can be found as supplemental material (online Supplementary Table S1). All articles retrieved were exported to the reference manager Mendeley (v1.19, Elsevier Inc.), where duplicate articles were removed. Additional publications were identified by hand-checking the reference lists of the relevant articles, searching for articles citing the relevant articles and through relevant review articles, but not through searching the grey literature.
Study selection and data extraction
Titles and abstracts of all studies were screened in Mendeley to assess whether they met the inclusion criteria (Table 2) for full-text review. If the decision on study inclusion or exclusion was unclear at this stage, the full text was obtained. Next, studies were further screened at the full-text level to ensure that they were eligible, were relevant to the research question and presented data in an appropriate manner. The screening process was performed independently by two reviewers at both the title and abstract phase (N. G. and A. M.) and at the full-text review (N. G. and D. C.-S.) in a systematic manner, with discrepancies resolved by a third reviewer if required (S. P.). Details of studies excluded at the full-text review with reason for exclusion are included in online Supplementary Table S2. Two review authors (N. G. and A. M.) then independently extracted key data into a prepared table, with discrepancies resolved by a third reviewer if required (S. P.). The authors were contacted if the full text was not available from our search. The following data were extracted from each study: country the study was performed in, study year, sample size, participant sex and mean age at follow-up (age range obtained if mean age unavailable), years of study follow-up, dietary assessment and statistical methods, whether dietary supplement use was reported or included in analysis, whether biochemical measures were included, whether nutrient intake was adjusted for energy or other measures, mean intake and standard deviation of nutrient intake at baseline and follow-up for each nutrient assessed, whether a significant difference was observed between nutrient intake at baseline and follow-up according to author’s statistical analysis. Details of the included studies’ funding sources and potential conflicts of interest are included in online Supplementary Table S3.
Table 2. Inclusion and exclusion criteria applied in article screening
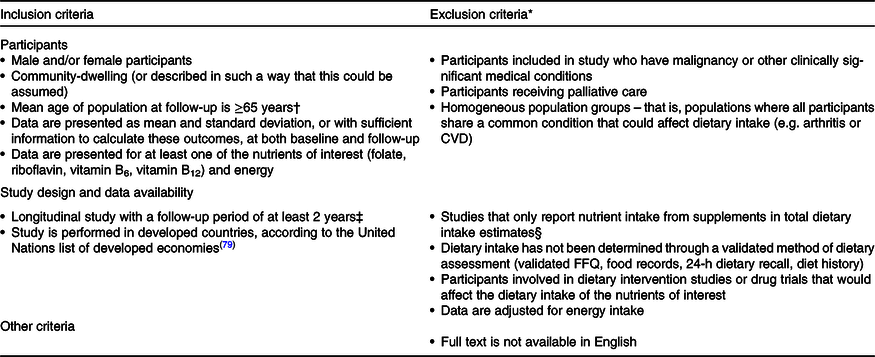
* Studies had to meet all inclusion criteria, and if any one additional exclusion criterion was met, the study was excluded from the final review.
† 65 years is the chronological age used in two commonly used indicators of ageing: the proportion of the population aged 65 years and older, and the old-age dependency ratio(Reference Sanderson and Scherbov80). The WHO also reports that most developed countries use the chronological age of 65 years as a definition for being an older person(81).
‡ A 2-year follow-up period was chosen to align with the minimum follow-up period of previous cohort studies (e.g. the Framingham Cohort(Reference Tucker, Selhub and Wilson63)) and to minimise the risk of detecting fluctuating changes in nutrient intake (e.g. with seasonality(Reference Shahar, Yerushalmi and Lubin82)) in shorter follow-up periods.
§ To strengthen conclusions that could be drawn around changes in dietary intake given the inconsistent reporting of supplement use in a prior systematic review of micronutrient intake in older adults(Reference ter Borg, Verlaan and Hemsworth25).
Quality assessment
A quality assessment scale applied by ter Borg et al.(Reference ter Borg, Verlaan and Hemsworth25), based on the Newcastle–Ottawa quality assessment scale for cohort studies(Reference Wells, Shea and O’Connell36), was adapted for use in review with additional criteria regarding the adequacy of follow-up of cohorts. A summary of criteria and point allocation for quality assessment is presented in Table 3. Summary quality scores of (0–2, 3–4 and 5–7) were rated as low, moderate and high, respectively. Studies were then categorised according to these ratings.
Table 3. Overview of the study quality assessment score
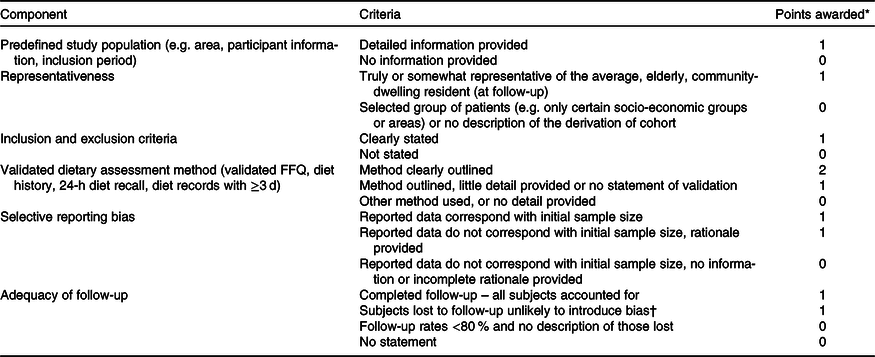
* Summary score: 0–2 points = low quality, 3–4 points = moderate quality, 5–7 points = high quality.
† Number lost is ≤20 %, or description of those lost suggested no different from those followed.
Statistical analysis
The population distribution of nutrient adequacy or inadequacy was based on the estimated average requirement (EAR) cut-point method. The cut-point approach determines the population prevalence of inadequate intakes as the proportion with intakes below the EAR, a value based on the intake level that is sufficient to meet the needs of 50 % of the population(37). As it is not appropriate to compare group means to the EAR in this approach(37), we first calculated z scores from the mean and standard deviation reported in each study. From here, intake distributions were calculated and segmented into frequencies of intake at percentages of the EAR. We can then apply the cut-point approach for risk of inadequacy by combining the total proportion of those estimated to have intakes below the EAR, as used in other reviews assessing micronutrient inadequacy on the mean and standard deviation of published data(Reference Roman Viñas, Ribas Barba and Ngo38,Reference Ter Borg, Verlaan and Mijnarends39) . This approach assumes that the requirement distribution is approximately symmetrical, and that intake distribution is more variable than the requirement distribution of the group(37). As nutrient reference values vary between countries, this was standardised in our analysis using the EAR from the Institute of Medicine on behalf of the USA and Canada(40). These recommendations align with nutrient reference values from Australia and New Zealand(41), but not with recommendations from the UK(42), or for many European countries where discrepancies in recommendations are well documented(Reference Doets, De Wit and Dhonukshe-Rutten43,Reference Pavlovic, Prentice and Thorsdottir44) .
Only one study(Reference Flood, Burlutsky and Webb45) required transformation for analysis, as dietary data were presented in the form of mean values with their standard error. Standard deviation was determined by applying the reported mean and 95 % CI using the following calculation for standard deviation(Reference Shuster46):
Results
Study selection and characteristics
Based on the search strategy, a total of 1579 articles were identified as potentially relevant, following the removal of duplicates retrieved across different databases.
After screening and eligibility assessment were completed, eight longitudinal studies were included in the final review (Fig. 1). This resulted in the inclusion of n 3119 (38 % male) community-dwelling older adults, with a range of n 78–1166 included participants and 2–25 years of follow-up in each study. The studies were conducted in seven developed countries: three articles from European countries, three from Australasia, one from North America and one from Asia (Table 4).

Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of article selection and inclusion.
Table 4. Characteristics of included studies assessing longitudinal dietary intake of folate and related vitamins in community-dwelling older adults
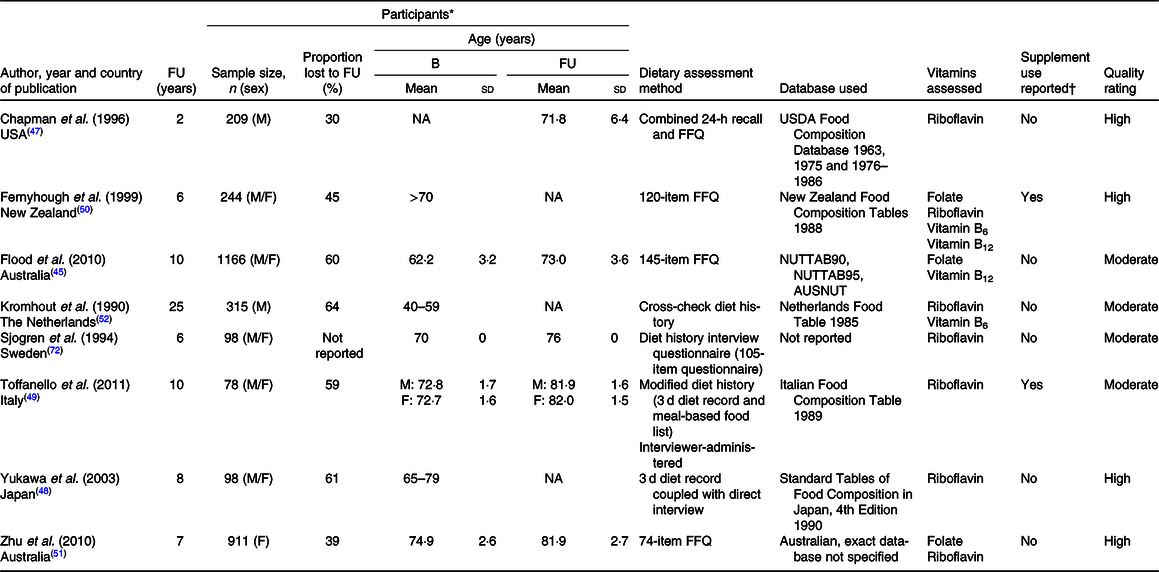
FU, follow-up; B, baseline; M, male; NA, not available; USDA, US Department of Agriculture; F, female.
* Age presented as mean and standard deviation unless stated otherwise if these values were not available. Sample size refers to the total number of participants included in both baseline and follow-up analyses for each study. Proportion of participants lost to follow-up includes the total of those no longer participating (e.g. due to death, illness or change in location), as well as those with incomplete dietary intake data in the follow-up surveys.
† Supplements were not included in the final analysis for any of the included studies that reported supplement use. It was assumed that if supplement use was not reported then supplements were not included in the final dietary analysis.
Dietary intake of riboflavin was assessed in seven studies (n 1953), folate in three studies (n 2321) and vitamins B6 and B12 both in two studies each (n 559 and 1410, respectively). None of the included studies assessed a particular group of micronutrients; rather, all studies looked at the change in wider nutrient intake. Habitual dietary intake was assessed by diet history methods (three studies), validated FFQ (three studies), combined 24-h recall and FFQ (one study) and a 3-d diet record (one study).
Overall, the risk of bias included in this review was relatively low – four studies were of high quality, four of moderate quality, while none of the included studies was found to be of low quality (Table 4).
Change in prevalence of dietary inadequacy with time
Riboflavin
The prevalence of nutritional inadequacy for riboflavin (i.e. those with dietary intakes that do not meet the EAR) was estimated to be greater than 25 % in three of the seven studies, and across both sexes at both baseline and follow-up(Reference Chapman-Novakofski, Ham and Pearlman47–Reference Toffanello, Inelmen and Minicuci49). At baseline the greatest prevalence of inadequacy was 43·9 and 36·3 %, while after follow-up it was 50·0 and 45·6 % in males and females, respectively. The magnitude of change in the prevalence of inadequacy from baseline to follow-up ranged from an increase of 0–22·6 and 0–9·3 % in males and females, respectively (Table 5).
Table 5. Calculated change in risk of micronutrient inadequacy between baseline (B) and follow-up (FU) of included studies
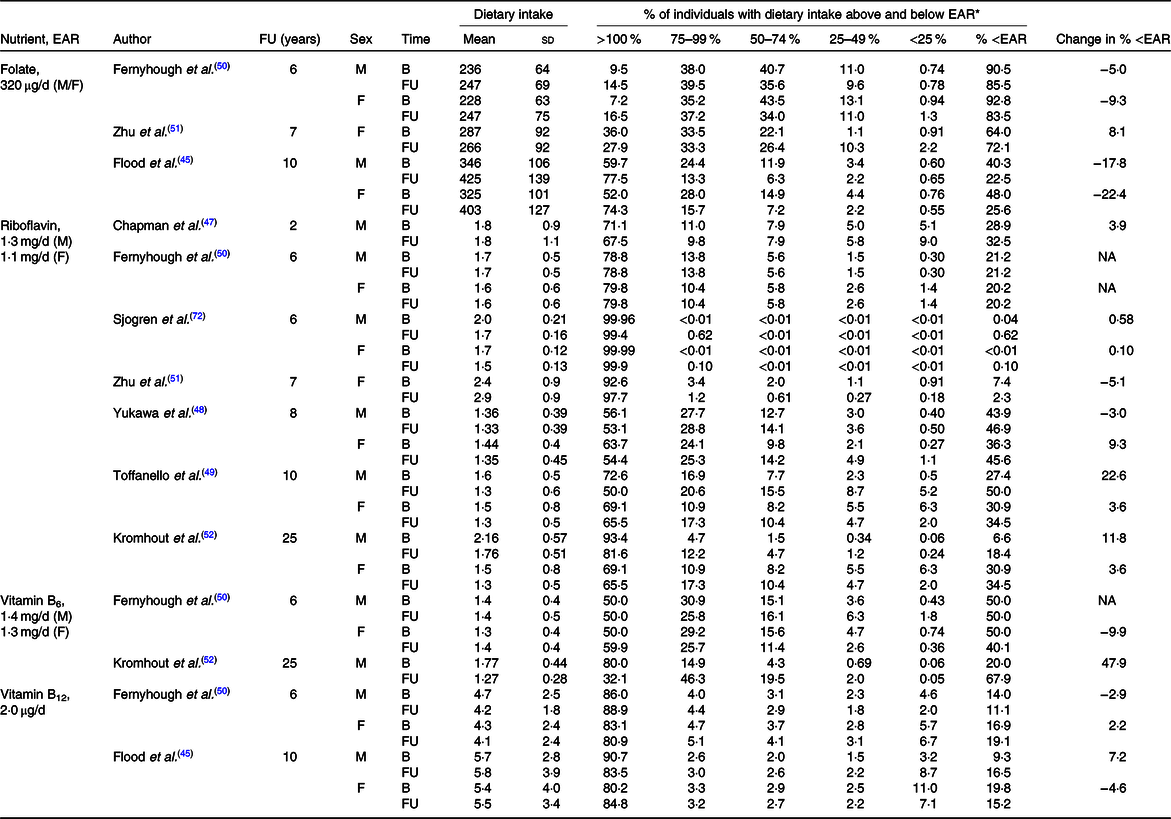
EAR, estimated average requirement; M, male; F, female; NA, not available.
* The population distribution of nutrient adequacy or inadequacy was based on the EAR cut-point method, using the EAR from the Institute of Medicine on behalf of the USA and Canada(40).
Folate
The prevalence of nutritional inadequacy for folate was estimated to be greater than 25 % in all three studies, and across both sexes at baseline and follow-up(Reference Flood, Burlutsky and Webb45,Reference Fernyhough, Horwath and Campbell50,Reference Zhu, Devine and Suleska51) . At baseline the greatest prevalence of inadequacy was 90·5 and 92·8 %, while after follow-up it was 85·5 and 83·5 % in males and females, respectively. The magnitude of change in prevalence of inadequacy from baseline to follow-up ranged from a decrease of 5·0–17·8 and 9·3–22·4 % in males and females, respectively, while only one study showed an increase in the prevalence of inadequacy of 8·1 % in females only (Table 5).
Vitamin B6
The prevalence of nutritional inadequacy for vitamin B6 was estimated to be greater than 25 % in one of the two studies, and across both sexes at baseline(Reference Fernyhough, Horwath and Campbell50), and in both studies across both sexes at follow-up(Reference Fernyhough, Horwath and Campbell50,Reference Kromhout, Coulander and Obermann-de Boer52) . At baseline the greatest prevalence of inadequacy was 50 % in both sexes, while after follow-up it was 67·9 and 40·1 % in males and females, respectively. The magnitude of change in prevalence of inadequacy from baseline to follow-up ranged from an increase of 0–47·9 % in males and a reduction of 9·9 % in females (Table 5).
Vitamin B12
The prevalence of nutritional inadequacy for vitamin B12 was not estimated to be greater than 25 % for either study at either baseline or follow-up(Reference Flood, Burlutsky and Webb45,Reference Fernyhough, Horwath and Campbell50) . At baseline the greatest prevalence of inadequacy was 14·0 and 19·8 %, while after follow-up it was 16·5 and 19·1 % in males and females, respectively. The magnitude of change in the prevalence of inadequacy from baseline to follow-up ranged from a 2·9 % reduction to a 7·2 % increase in males and from a 4·6 % reduction to a 2·2 % increase in females(Reference Flood, Burlutsky and Webb45,Reference Fernyhough, Horwath and Campbell50) (Table 5).
Discussion
Older adults are a vulnerable group with respect to micronutrient intake and status owing to a complex interplay of physiological and psycho-sociological factors intrinsic to ageing, leading to the notion that micronutrient intake declines with advancing age. However, reports on nutrient intake in community-dwelling older adults vary widely and rarely describe what changes occur due to ageing as opposed to the frequently reported cross-sectional differences in dietary intake compared with other age groups. The current systematic review presents the first pooled analysis of changes in micronutrient intake that occur due to the ageing process, providing insights into how dietary intake might be optimised in ageing populations. From eight longitudinal studies, we found evidence of decreased dietary adequacy of riboflavin with age, as well as a high prevalence of dietary inadequacy of folate, riboflavin and vitamin B6. More importantly, we have highlighted the extreme scarcity of literature available to substantiate the often reported claim that micronutrient intake declines due to ageing.
From the eight studies included in our analysis, evidence for changes in dietary adequacy was limited for folate (three studies) and vitamins B6 (two studies) and B12 (two studies). Indeed, we could not identify a trend for changes in dietary adequacy of these nutrients. Dietary riboflavin was most frequently reported, and dietary adequacy was found to decline into older age in five of the seven studies reporting on riboflavin. This decline supports the cross-sectional meta-analysis of dietary intakes in older adults by ter Borg et al. (Reference ter Borg, Verlaan and Hemsworth25), which identified riboflavin as a key nutrient of concern in older adults with an estimated prevalence of inadequacy of 31–41 % across thirty studies. One study in the current review reported an increase in both occasional and regular multivitamin supplement use from baseline to follow-up, particularly in females(Reference Fernyhough, Horwath and Campbell50), which although not considered in our analysis could of course mitigate the decline in riboflavin adequacy. Although another study reported that participants were not taking supplements at baseline, they did not report follow-up data on whether habits around supplement intake changed with time(Reference Toffanello, Inelmen and Minicuci49), and the other five studies did not report on supplement use. Thus, it cannot be presumed that the decline in dietary riboflavin adequacy reported in this review reflects the total nutrient adequacy from food and supplements. It was not possible to disentangle changes in dietary adequacy from supplement use in the current review due to inconsistencies in reporting of supplement use, which as previously criticised by ter Borg et al. (Reference ter Borg, Verlaan and Hemsworth25) limits our insight into the true prevalence of micronutrient inadequacies in older adults. The present study supports demand for improved reporting of dietary intake data including supplement sources of nutrients from such studies to better understand changing dietary habits and status with advancing age. In the current review, original authors attributed the change in riboflavin intake to a significant reduction in energy intake, with smaller portions consumed(Reference Zhu, Devine and Suleska51), a reduced intake of milk products(Reference Zhu, Devine and Suleska51), meat or fish(Reference Toffanello, Inelmen and Minicuci49) and an increased preference for sweet items with age(Reference Toffanello, Inelmen and Minicuci49). Thus, ways to promote these and other widely available dietary sources of riboflavin (e.g. fortified cereals) throughout ageing should be considered in dietary recommendations and clinical practice to ensure nutritional adequacy with improved health outcomes for older adults.
Evidence for longitudinal changes was limited to eight studies including just 3119 participants; irrespective of limited evidence, this review highlighted folate, riboflavin and vitamin B6 dietary inadequacy as a potential concern with advancing age. Across the studies included, the prevalence of dietary inadequacy at follow-up was estimated to be greater than 25 % for studies reporting on riboflavin (three studies), folate (three studies) and vitamin B6 (two studies), though the prevalence of inadequate vitamin B12 intake was comparatively lower at less than 20 %. Estimates of the prevalence of dietary inadequacy varied widely in our analysis, particularly in the case of riboflavin, which ranged from 0 to 43·9 % at baseline and from 0 to 50 % at follow-up, and the results must be interpreted accordingly with some caution. Yet, this longitudinal analysis supports similar cross-sectional findings of micronutrient adequacy in older adults(Reference ter Borg, Verlaan and Hemsworth25), as riboflavin, folate and vitamin B6 were reported to have an estimated prevalence of inadequacy of greater than 25 % across thirty studies, while the prevalence of B12 inadequacy was less than 20 %. Understanding longitudinal changes provides context to such cross-sectional analyses, helping to characterise their relevance to appropriate recommendations. Indeed, our analysis showed that the decline in dietary riboflavin adequacy was progressive (up to a 22·6 and 9·3 % increase in dietary inadequacy in males and females, respectively), highlighting the need to consider dietary intervention even prior to old age.
Factors contributing to changes in dietary intake with age are well established, ranging from physiological, such as sensory changes or increasing disability, to socio-economic pressures and psychological perturbations(Reference Morley7,Reference Morley8) . While this review highlights that a progressive decline in dietary adequacy of riboflavin may occur with ageing, intake is only a single facet required to understand micronutrient status and function in pathways like one-carbon metabolism with advancing age. Physiological functions that influence nutrient status are also known to be altered in older age including impaired absorption(Reference Morley8), low bioavailability from foods(53) and the impact of genetic polymorphisms on micronutrient status and function(Reference Agostoni, Berni Canani and Fairweather-Tait54,Reference Höller, Bakker and Düsterloh55) . Such factors have led to controversy over the appropriateness of dietary reference values for older age, as these values, which are largely extrapolated from younger reference populations(Reference Doets, De Wit and Dhonukshe-Rutten43,Reference Pavlovic, Prentice and Thorsdottir44) , have been suggested to contribute to discrepancies reported between dietary intake and nutrient status(Reference Bates, Prentice and Cole56). Although direct correlation between the intake and status of all B vitamins is not robustly established in older adults(Reference Bates, Prentice and Cole56), concerns regarding the correlation between vitamin B12 intake and status in older adults serve to highlight the confounding influence of complex digestive processes on inferring adequacy from intake alone. Compromised vitamin B12 status is observed in older adults despite dietary adequacy due to progressive changes to the gastrointestinal tract with ageing that impair its absorption(Reference Hughes, Ward and Hoey57,Reference Allen58) . Accordingly, while vitamin B12 intake was largely adequate in our analysis, this does not rule out the possibility of a low biochemical status that would contribute to functional decline. Although we primarily aimed to characterise changes in dietary adequacy with age, it is worthwhile to note that, as far as we are aware, only one study included in the current review reported on longitudinal changes in both B vitamin intake and biochemical measures of B vitamin status(Reference Chapman-Novakofski, Ham and Pearlman47). Hence, there remains a poor understanding of how changes in dietary adequacy with advancing age reported in this review extend to determine biological micronutrient status and health risk.
Despite the wealth of robustly studied longitudinal cohorts following participants into advancing age(Reference Willett and Stampfer59–Reference Collerton, Barrass and Bond62), our search for longitudinal dietary changes in a group of B vitamins resulted in only eight studies included in the final review, and a total sample size of only 3119 participants. Unfortunately, a number of well-characterised, large, prospective cohorts did not meet the inclusion criteria for this review despite identification through our search strategy. The reasons included longitudinal data not being available for nutrients of interest (e.g. the Framingham Heart Study(Reference Tucker, Selhub and Wilson63–Reference Tucker, Rich and Rosenberg65)), not meeting criteria for age at follow-up, inadequate presentation of nutrient intake (the Nurses’ Health Study, the Health Professionals Follow-Up Study(Reference Lee, Willett and Fuchs66)) or the full text not being available after request (e.g. the SENECA study(Reference Amorim Cruz, Moreiras and Brzozowska67)). While these studies could not be included in our final review, the longitudinal data published from the Nurses’ Health Study and Health Professionals Follow-Up Study(Reference Lee, Willett and Fuchs66) would suggest that folate intake from both natural food sources and fortified foods increases with age, although the participants did not reach older age by the time of follow-up. Data on B vitamin intake in the Framingham Cohort have only been published at the 20th follow-up(Reference Tucker, Selhub and Wilson63–Reference Tucker, Rich and Rosenberg65), and although not able to be combined to show longitudinal trends, cross-sectional data in this cohort (n 1160)(Reference Selhub, Jacques and Rush64) would also suggest that folate and vitamin B12 intake increases concurrently with categories of age. Both of these studies contrast the current understanding that micronutrient intake likely declines with advancing age, while the data synthesised from this review are equivocal given the paucity of longitudinal intake data reported on folate and vitamin B12 intake. Evidently, better or further reporting of longitudinal nutrient intake is needed, particularly in these large cohorts where the data are likely available. This would help to better characterise the unique nutrient changes and requirements that occur with advancing age rather than relying on cross-sectional reports.
Although the included studies were all of moderate–high quality, participants were often not representative of the wider community-dwelling older population, tending to be of higher socio-economic and health status(Reference Flood, Burlutsky and Webb45,Reference Zhu, Devine and Suleska51) , which is likely to be reflected in dietary intake(Reference Si Hassen, Castetbon and Cardon68,Reference Zhang, Hsu-Hage and Wahlqvist69) . Indeed, dietary assessment methodology was robust for all included studies (diet history, diet records, validated FFQ), but the variation of methods limits the comparison of dietary intake between studies included in this review. Adjusting nutrient intake for energy can help to address differences in dietary assessment methodology(Reference Willett70); however, this was not possible in the current review as we did not have access to raw data. Further, it should be acknowledged here that the EAR cut-point approach is subject to over-inflation of prevalence estimates, and we were unable to adjust for within-person variation in nutrient intakes; thus, our results may overestimate the prevalence of inadequacy(Reference Taylor, Carriquiry and Bailey71). We were unable to adequately comment on sex differences in age-related changes in intake, as several studies included only one sex or provided conflicting findings. Similarly, we were unable to conduct sub-analyses of nutrient intake according to supplement use, as only two studies reported on supplement users, and this was not included in the final analysis. Additionally, although studies investigated changes in general micronutrient intake, the nutrients included were inconsistent, without strong rationale for inclusion or exclusion. This limited and inconsistent reporting restricts the strength of conclusions and recommendations from the current review, particularly around dietary intake of folate and vitamins B6 and B12, reported in less than half of included studies. Further, as follow-up varied from 2 to 20 years, and there were no clearly defined age groups from the included studies, we are unable to draw contrasts across either different lengths of follow-up or ageing groups.
Longitudinal studies, while useful for describing progressive changes in dietary adequacy, are subject to the confounding influence of changes in intake naturally occurring over time; changes in the food supply or population dietary recommendations and behaviours may influence shifts in an individual’s intake. Three studies in this review attempted to account for this and conducted time-sequential analyses by including cohort comparisons ranging from 6 to 25 years(Reference Fernyhough, Horwath and Campbell50,Reference Kromhout, Coulander and Obermann-de Boer52,Reference Sjögren, Österberg and Steen72) . The reported changes in intake were inconsistent; some authors found increased dietary intake over time of riboflavin(Reference Sjögren, Österberg and Steen72), vitamin B6 (Reference Fernyhough, Horwath and Campbell50) and folate(Reference Fernyhough, Horwath and Campbell50), while another author found decreased vitamin B6 (Reference Sjögren, Österberg and Steen72) intake. Given the paucity of data, these analytical corrections are not sufficiently robust to influence the conclusions drawn from this review. Moreover, the effect of common population-wide influences limits the accuracy of estimating shifts in an individual’s nutrient intake over time. This includes factors such as dietary assessment methods and differences in databases used between studies, as well as progressive changes to databases, for which corrections could not be made(Reference Sempos, Flegal and Johnson73). In particular, the limitations of comparing dietary folate intake between countries are widely acknowledged, stemming from differences in how different folate forms (naturally occurring folate and fortified folic acid) are estimated, and whether databases are up to date at the time of dietary analysis(Reference Troesch, Hoeft and McBurney4,Reference Bouckaert, Slimani and Nicolas74,Reference Deharveng, Charrondière and Slimani75) . For example, studies reporting on folate intake in the current review(Reference Flood, Burlutsky and Webb45,Reference Fernyhough, Horwath and Campbell50,Reference Zhu, Devine and Suleska51) use food composition databases (Australian (AUSNUT, NUTTAB90, NUTTAB95)(Reference Flood, Burlutsky and Webb45,Reference Zhu, Devine and Suleska51) , New Zealand Food Composition Tables(Reference Fernyhough, Horwath and Campbell50)) that take folic acid voluntarily fortified by the industry (e.g. breakfast cereals) into account. Further, data in New Zealand(Reference Fernyhough, Horwath and Campbell50) are largely based on the manufacturer’s claims which may not reflect the true analytical value(76). This is further compounded by changing folate fortification policies over time within countries. None of the studies reporting on changes in folate intake in this review was conducted in countries (Australia(Reference Flood, Burlutsky and Webb45,Reference Zhu, Devine and Suleska51) and New Zealand(Reference Fernyhough, Horwath and Campbell50)) at a time (between 1988 and 2006) where folic acid fortification was mandatory (only since 2009 in Australia(77), voluntary since 2009 in New Zealand(78)). However, changes in voluntary folate fortification practices in Australia were suggested by Flood et al. (Reference Flood, Burlutsky and Webb45) to be the reason why increasing folate intake was seen. Thus, it remains difficult to disentangle the effects of time and age on intake.
This systematic review provides evidence for alterations in dietary adequacy of B vitamins with the ageing process, including a progressive decline in riboflavin adequacy and a high prevalence of inadequacy for folate, riboflavin and vitamin B6, which complements and expands upon previous cross-sectional analyses. A limited number of studies and participants were included in this review, emphasising the lack of understanding around changes in nutrient intake with ageing, in contrast to the knowledge of differences in intake across age groups. Although supporting concerns around inadequate micronutrient intake in older adults, these concerns remain entangled with potential changes in overall dietary intake or micronutrient bioavailability and metabolism. To understand the true implications of these findings on related health outcomes and contribution to disease burden in ageing, a complementary understanding of concurrent biological changes to nutrient status is required. Hence, although this review of changes in dietary intake is informative on the risk of subclinical malnutrition in older adults, further evidence is required from longitudinal research including comprehensive assessment of intake and markers of biochemical and functional status to direct micronutrient recommendations for older adults.
Acknowledgements
N. G. was supported by a University of Auckland Doctoral Scholarship, a Hope Foundation Scholarship, and by AgResearch Limited through the Strategic Science Investment Fund (Contract A21246: Nutritional strategies for an ageing population) while completing this work.
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
N. G. formulated the research question, was responsible for study design, data screening and synthesis, and writing the manuscript. A. M. M. and D. C. S. were responsible for data screening and critical revision of the manuscript. C. R. W. was responsible for critical revision of the manuscript. S. P. provided guidance on systematic review methodology and reviewed the manuscript.
There are no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004249