Abnormal hepatic lipid deposition, excessive TAG accumulation in the liver, is increasingly prevalent in the farmed fish, due to the wide use of high-fat diet (HFD) which has a cost-effective farming and protein-sparing effects(Reference Boujard, Gélineau and Covès1). Hepatocyte lesion and lower disease resistance are indicators of hepatic steatosis caused by abnormal hepatic lipid deposition, resulting in considerable losses in aquaculture(Reference Du, Clouet and Huang2,Reference Yan, Liao and Wang3) . Collectively, it is meaningful to study lipid metabolism and explore a way to mitigate the negative effects of hepatic steatosis in the farmed fish.
Curcumin (CC, commonly known as turmeric), a polyphenolic compound derived from the Curcuma longa, is well documented for its medicinal properties in Chinese and Indian medicine systems(Reference Aggarwal, Takada and Oommen4,Reference Aggarwal, Kumar and Bharti5) . Its biological and pharmacological functions have been well confirmed, such as antioxidative(Reference Ruby, Kuttan and Babu6), anti-inflammatory(Reference Jurenka7) and antimicrobial(Reference Han and Yang8). Additionally, recent studies have found that CC could alleviate liver diseases(Reference Nabavi, Daglia and Moghaddam9,Reference Nanji, Jokelainen and Tipoe10) and CVD(Reference Aggarwal and Harikumar11) by regulating lipid and cholesterol metabolism(Reference Nanji, Jokelainen and Tipoe10–Reference Shin, Ha and McGregor13). Meanwhile, CC plays a vital role in the obesity and metabolic diseases treatment through mediating lipid metabolism and inflammatory responses(Reference Shehzad, Ha and Subhan14–Reference Ejaz, Wu and Kwan17). Given the medicinal properties and healthy benefits of CC in mammals, CC has attracted significant attention to aquaculture. CC and its active molecule have been confirmed to exert positive effects on fish health and lipid metabolism, including hepatoprotective, anti-inflammatory, immunomodulatory, antioxidant and antistress(Reference Abdel-Tawwab and Abbass18–Reference Mahmoud, Al-Sagheer and Reda20). In tilapia, 50 mg/kg CC remarkably improved growth performance and immunity(Reference Mahmoud, Al-Sagheer and Reda20). Fish fed the diet supplemented with 5 g/kg CC showed significantly higher final body weight and antioxidant capacity in crucian carp(Reference Jiang, Wu and Zhou21). Therefore, CC has the potential to mitigate the adverse effects caused by HFD in farmed fish. In addition, plant species-based additives have no or fewer residue concerns and the adverse effects on animal, human and environment health(Reference Mahmoud, Al-Sagheer and Reda20,Reference Baba, Acar and Öntaş22) , as potential growth and health promoter in aquaculture(Reference Sutili, Gatlin and Heinzmann23,Reference Chakraborty, Horn and Hancz24) .
Large yellow croaker (Larimichthys crocea), widely cultured in southeast China, is an important economical marine fish due to its delicious taste and high nutritional value(Reference Liu and De Mitcheson25). Furthermore, lipid metabolisms among the large yellow croaker, other fish and mammals are similar, and large yellow croaker could be considered as an appropriate model for regulation of lipid metabolism in marine fish(Reference Yan, Liao and Wang3,Reference Cai, Feng and Xiang26–Reference Ji, Xu and Xiang28) . Thus, this study was to investigate the protective effects of CC against hepatic steatosis. The underlying mechanism of the beneficial effect of CC was also focused.
Materials and methods
Animal ethics
The present study was conducted in strict accordance with the Management Rule of Laboratory Animals (Chinese Order No. 676 of the State Council, revised 1 March 2017) and approved by the Institutional Animal Care and Use Committee of the Ocean University of China.
Feed ingredients and diet formulation
Ingredients and nutrient composition of four experimental diets are shown in online Supplementary Table S1. Four isoproteic (43 % crude protein) and isolipidic (18 % crude lipid) diets were formulated to contain graded levels of CC (0, 0·02, 0·04 and 0·06 %; Beijing Solarbio Technology Co. Ltd; concentration ≥ 95 %) (online Supplementary Table S1). The diet (18 % lipid content) without CC supplementation was the control diet. Previous studies have been confirmed that 18 % lipid level diet had the negative impacts on large yellow croaker, resulting in abnormal hepatic lipid deposition and inflammatory response(Reference Yan, Liao and Wang3,Reference Wang, Yan and Xu29) .
Dietary ingredients were ground into fine powder through a 320 μm mesh. All the ingredients were thoroughly blended first by hand and then by machine. After that, all ingredients were thoroughly mixed with oil mixture and water, respectively. The automatic fish granulator (F-26; South China University of Technology) was used to make pellets (4 mm × 5 mm and 5 mm × 5 mm. Pellets were dried for about 12 h in an oven at 55°C, placed in double plastic bags and stored at −20°C until the trial.
Experimental procedure
Juvenile large yellow croaker was provided by the Fu Fa Aquatic Products Co. Ltd. Before the start, fish were reared in floating sea cages (2 m × 4 m × 2 m) and fed the control diet for 14 d to adapt to the experimental conditions and diets.
At the initiation, fish were starved for 24 h and weighed. The fish of similar sizes (15·92 (sem 0·16) g) were distributed into twelve sea cages (1 m × 1 m × 2 m). Each diet was randomly allocated to three cages (sixty fish/each cage) and fed twice daily (05.00 and 17.00 hours) for 10 weeks. During the experiment, conditions were as followed: water temperature (26·5–31·0°C), salinity (32–36‰) and dissolved oxygen (approximately 7 mg/l).
Sample collection
At the termination, fish were starved for 24 h and anaesthetised with MS222 (1:10 000; Sigma). The body weight and total number of fish in each cage were recorded for analyses of survival rate, final body weight, weight gain and specific growth rate. Six fish (each cage) were collected to analyse whole-body composition. The wet weight of the body, liver and visceral of six fish (per cage) was measured to analyse morphometric parameters. Muscle and liver tissue (at least six fish/cage) were collected and mixed for fatty acid profile and lipid content analyses, respectively. The blood was obtained from the caudal vein by 1 ml syringes from ten fish (each cage) and placed to clot at 4°C for 6–8 h. The clot and residual blood cells were removed by centrifugation for 10 min (3500 r/min). The serum was placed at −80°C for later analysis of biochemical and antioxidant parameters. Liver tissue (nine fish/each cage) was collected and mixed in 1·5 ml RNA-free tubes and then were immediately frozen in liquid N2 which were stored at −80°C for gene expression analyses.
Proximate composition, fatty acid profile and curcumin analyses
Crude protein, crude lipid and moisture of the whole body and the diet were analysed following the procedures of the Association of Official Analytical Chemists (AOAC, 2005). Briefly, the moisture content was measured by drying to constant weight at 105°C for 6–8 h. The crude lipid and crude protein content (N × 6·25) were determined by the ether extraction using Soxhlet method and Kjeldahl method, respectively. Lipid contents of liver and muscle were extracted and measured by chloroform–methanol (v/v, 2:1) as previously described(Reference Folch, Lees and Sloane Stanley30).
The fatty acid profile of liver and muscle was determined using the previously described procedures(Reference Metcalfe, Schmitz and Pelka31) with some modification(Reference Zuo, Ai and Mai27,Reference Ai, Zhao and Mai32,Reference Ji, Li and Li33) . The liver and muscle tissues were freeze-dried in a lyophilized chamber (Alpha 1-4 LDplus; Christ). HP6890 gas chromatograph (Agilents Technologies Inc.) with the capillary column (007-CW; Hewlett Packard) and a flame ionization detector were used to identify and quantify fatty acid methyl esters.
CC was analysed using the previously described procedures(Reference Li, Jiang and Wen34) and National Standards of the People’s Republic of China (GB1886.76-2015) with some modification. Liquid chromatographic conditions: Purospher C18 column (4·6 mm × 250 mm, 5 μm particle size; Merck Drugs & Biotechnology). The mobile phase was composed of acetonitrile–4 % acetic acid (55:45, v/v) at a flow rate of 1·0 ml/min. The wavelength of the detection was at 324 nm. The column temperature was 35°C. The injection volume was 10 μl.
Serum parameters and antioxidant capacity analysis
Serum total cholesterol, TAG, LDL-cholesterol, HDL-cholesterol, alanine transaminase and aspartate transaminase were determined by the automatic biochemical analyzer (Mindry BS-180; Mindry).
Serum superoxide dismutase (SOD), catalase (CAT), total antioxidant capacity and malondialdehyde (MDA) were measured by a commercial kit (Nanjing Jiancheng Bio-Engineering Institute). SOD activity was measured by the xanthine/xanthine oxidase method based on the production of O2−, which oxidises hydroxylamine to form nitrites, appearing purplish red in the presence of chromogenic agents. The CAT decomposition of H2O2 is quickly terminated by adding ammonium molybdate. The remaining H2O2 forms a yellow complex with ammonium molybdate, and its variation is measured at 405 nm to calculate the CAT activity. Total antioxidant capacity is determined by colourimetry, where antioxidant substances reduce Fe3+ to Fe2+, and Fe2+ and phenanthrene substances form stable complexes. MDA is determined by the thiobarbituric acid method, where MDA and thiobarbituric acid form a red product with a maximum absorbance value at 532 nm.
Quantitative real-time PCR
Gene expression was determined by real-time reverse-transcriptase quantitative PCR according to the previous procedure(Reference Ji, Li and Li33). Total RNA was extracted from the liver of large yellow croaker using a Trizol Reagent (Invitrogen). The quality of isolated RNA was measured spectrophotometrically (Nanodrop 2000; Thermo Fisher Scientific), and the integrity was determined on a 1·2 % denaturing agarose gel. Then, RNA was reverse-transcribed to cDNA by a Prime Script-RT reagent Kit with gDNA Eraser (perfect real-time) (Takara). Reverse-transcriptase quantitative PCR was performed in a quantitative thermal cycler (CFX96™ Real-Time System; BIO-RAD). The amplification was performed in a total volume of 25 μl, containing 1 μl of each primer (10 μmol), 1 μl cDNA product, 12·5 μl SYBR-Premix ExTaqII (Takara) and 9·5 μl RNA-free water. The real-time PCR programme was as follows: 95°C for 2 min, followed by forty cycles of 95°C for 10 s, 58°C for 10 s and 72°C for 20 s. The primer sequences were obtained from previous paper(Reference Yan, Liao and Wang3,Reference Cai, Feng and Xiang26,Reference Ji, Xu and Xiang28,Reference Li, Monroig and Wang35) (online Supplementary Table S2). The amplification efficiencies of all genes were approximately equal and ranged from 94 to 105 %. The mRNA levels were calculated using the 2−ΔΔt method(Reference Livak and Schmittgen36). Finally, data were expressed as fold change relative to the control group.
Statistical analysis
SPSS 19.0 (SPSS) was used to analyse all data. Data are shown as mean values with their standard errors. The one-way ANOVA method was used to analyse all data. Statistical significance was set at P < 0·05 using Tukey’s multiple range tests.
Results
Growth performance and survival rate
Final body weight, weight gain and specific growth rate in fish fed the diet with 0·04 % CC were significantly higher than the control group (fish fed the HFD) (P < 0·05) (Table 3). There were no statistical differences in SR, hepato-somatic index and viscera-somatic index among treatment groups (P > 0·05) (Table 1).
Table 1. Growth response and survival of large yellow croaker fed diets with graded levels of curcumin level (n 3) (Mean values with their standard errors)

WG, weight gain; SGR, specific growth rate; SR, survival rate; HSI, hepato-somatic index; VSI, viscera-somatic index.
a,b Mean values within a row with the same superscript letters were not significantly different among dietary treatments by Tukey’s test (P > 0·05).
* WG (%) = 100 × (final weight − initial weight)/initial weight.
† SGR (%/d) = 100 × (Ln final weight − Ln initial weight)/experiment period.
‡ SR (%) = 100 × final number/initial number.
§ HSI (%) = 100 × liver weight/body weight.
|| VSI (%) =100 × visceral weight/body weight.
Whole-body composition and lipid deposition in liver and muscle
The lipid levels of the whole body and liver were dramatically affected by dietary CC (P < 0·05) (Table 2). Liver lipid content decreased dose-dependently with increased CC, where liver lipid levels of 0·04 and 0·06 % CC groups were remarkably lower than the control group (P < 0·05). In the whole body, fish fed the diet with 0·04 % CC had the lowest lipid content (P < 0·05). No significant differences were observed in crude protein, moisture and muscle lipid content among treatment groups (P > 0·05) (Table 2).
Table 2. Effects of curcumin on whole body composition of large yellow croaker (n 3) (% wet weight)(Mean values with their standard errors)
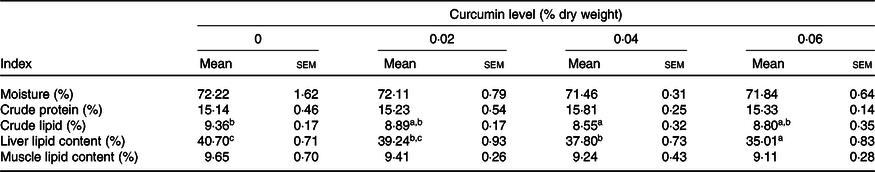
a,b,c Mean values within a row with the same superscript letters were not significantly different among dietary treatments by Tukey’s test (P > 0·05).
Serum metabolite parameters
The levels of total cholesterol, TAG and LDL-cholesterol decreased dose-dependently with the increased CC (Fig. 1). Fish fed diets with 0·04 and 0·06 % CC had markedly lower levels of LDL-cholesterol and TAG than the control group (P < 0·05) (Fig. 1), while HDL-cholesterol levels were significantly higher in fish fed the diet with 0·04 % CC (P < 0·05) (Fig. 1). The activity of alanine transaminase and aspartate transaminase was significantly decreased in 0·04 % CC group compared with the control group (P < 0·05) (Fig. 1).

Fig. 1. Serum biochemical indexes and enzyme activities. (A) TAG, (B) total cholesterol (TC), (C) LDL-cholesterol, (D) HDL-cholesterol, (E) alanine transaminase (ALT) and (F) aspartate transaminase (AST) of large yellow croaker fed experimental diets. Results are means with their standard errors (n 3). a,b,c Mean values with the same letters were not significantly different among dietary treatments by Tukey’s test (P > 0·05).
Antioxidant parameters
MDA content was remarkably lower in CC supplementation groups compared with the control group (P < 0·05) (Fig. 2); moreover, the lowest MDA was observed in fish fed the diet with 0·04 % CC (P < 0·05). The activity of CAT and SOD was strikingly higher in CC groups than the control group. In addition, fish fed the diet with 0·04 % CC had the highest activity of SOD and CAT (P < 0·05) (Fig. 2). Similarly, fish fed diets with 0·04 % and 0·06 % CC had significantly higher total antioxidant capacity than the control group (P < 0·05) (Fig. 2).

Fig. 2. Antioxidant parameters in serum: (A) malondialdehyde (MDA), (B) superoxide dismutase (SOD), (C) catalase (CAT) and (D) total antioxidant capacity (T-AOC) in serum of large yellow croaker fed experimental diets. Results are means with their standard errors (n 3). a,b,c,d Mean values with the same letters were not significantly different among dietary treatments by Tukey’s test (P > 0·05).
Fatty acid profiles
The ratio of hepatic SFA:C18 : 1n-9 decreased gradually with the increased CC. However, C20 : 4n-6, C20 : 5n-3, C22 : 6n-3, n-6 PUFA and n-3 PUFA increased dose-dependently when CC increased (Table 3). In the liver, SFA and C18 : 1n-9 were significantly lower in 0·04 % CC group than the control group (P < 0·05). The C20 : 4n-6, C20 : 5n-3, C22 : 6n-3, n-3 PUFA, n-6 PUFA, n-3:n-6 PUFA and n-3 Lc-PUFA were markedly higher in fish fed the diet with 0·04 % CC than the control group (P < 0·05). There was an upward trend of C22 : 6n-3, n-3 PUFA, n-3:n-6 PUFA and n-3 Lc-PUFA in the muscle, and no significant differences were observed among treatment groups (P > 0·05) (Table 4).
Table 3. Fatty acid profiles (% total fatty acids) in the liver of large yellow croaker (n 3)(Mean values with their standard errors)

LC-PUFA, long-chain PUFA.
a,b,c Mean values within a row with the same superscript letters were not significantly different among dietary treatments by Tukey’s test (P > 0·05).
Table 4. Fatty acid profiles (% total fatty acids) in the muscle of large yellow croaker (n 3)(Mean values with their standard errors)

LC-PUFA, long-chain PUFA.
mRNA levels of genes related to lipid metabolism
The transcript levels of pparα, carnitine palmitoyltransferase I (cpt1) and acyl-CoA oxidase (aco) notably increased in CC treatment groups compared with the control group (P < 0·05); furthermore, fish fed the diet with 0·04 % CC had the highest expression of pparα, cpt1 and aco (P < 0·05) (Fig. 3(A)). The mRNA levels of sterol-regulatory element-binding protein 1 (srebp1) and fatty acid synthase (fas) in CC treatment groups dramatically decreased compared with the control group (P < 0·05). No significant differences were observed in diacylglycerol O-acyltransferase 2 (dgat2) mRNA levels among dietary treatment groups (P > 0·05) (Fig. 3(B)). The three genes, consisting of elongation of very long-chain fatty acids protein 4 (elovl4), elovl5 and Δ6 fatty acyl desaturase (Δ6fad), were involved in the LC-PUFA biosynthetic pathway. These genes significantly up-regulated in CC treatment groups compared with the control group (P < 0·05), and fish fed the diet with 0·04 % CC had the highest expression of elovl4 and elovl5 (Fig. 3(C)).

Fig. 3. Effects of dietary curcumin (CC) on relative mRNA levels of genes involved in lipid metabolism pathways including (A) catabolism, (B) anabolism and (C) long-chain PUFA biosynthesis in the liver of large yellow croaker. Results are means with their standard errors (n 3). a,b,c,d Mean values with the same letters were not significantly different among dietary treatments by Tukey’s test (P > 0·05). ![]() , 0 % CC;
, 0 % CC; ![]() , 0·02 % CC;
, 0·02 % CC; ![]() , 0·04 % CC;
, 0·04 % CC; ![]() , 0·06 % CC. aco, Acyl-CoA oxidase; cpt1, carnitine palmitoyltransferase I; srebp1, sterol-regulatory element-binding protein 1; fas, fatty acid synthase; dgat2, diacylglycerol O-acyltransferase 2; elovl4, elongation of very long-chain fatty acids protein 4; elovl5, elongation of very long-chain fatty acids protein 5; Δ6fad, Δ6 fatty acyl desaturase.
, 0·06 % CC. aco, Acyl-CoA oxidase; cpt1, carnitine palmitoyltransferase I; srebp1, sterol-regulatory element-binding protein 1; fas, fatty acid synthase; dgat2, diacylglycerol O-acyltransferase 2; elovl4, elongation of very long-chain fatty acids protein 4; elovl5, elongation of very long-chain fatty acids protein 5; Δ6fad, Δ6 fatty acyl desaturase.
Discussion
HFD have been widely used due to cost-effective farming and protein-sparing effects in aquaculture(Reference Boujard, Gélineau and Covès1). Suitable feed additives are effective to alleviate the adverse effects of HFD in farmed fish. The recent studies have found that dietary CC supplementation increased protein content and inhibited lipid peroxidation in the climbing perch liver(Reference Manju, Sherin and Rajasekharan37,Reference Manju, Akbarsha and Oommen38) . In other fish, 50 mg/kg CC significantly improved the growth performance of tilapia fed the standard diet(Reference Mahmoud, Al-Sagheer and Reda20). Fish fed the diet with 5 g/kg CC showed remarkably higher final body weight of crucian carp(Reference Jiang, Wu and Zhou21). Previous studies have been confirmed that 18 % lipid levels diet (HFD) could cause hepatic lipid deposition with lower antioxidant capacity and immunity in large yellow croaker(Reference Yan, Liao and Wang3,Reference Wang, Yan and Xu29,Reference Yi, Zhang and Xu39) . This study found that 0·04 % CC supplementation markedly improved the growth performance of large yellow croaker fed HFD. Therefore, these suggested that CC could mitigate the adverse effects of HFD and further promote the growth of large yellow croaker.
The present study showed that lipid levels of the whole body and liver significantly decreased in 0·04 % CC group, indicating that CC had a lipid-lowering effects on fish. Similar results were found in mammals that CC plays a role in lipid lowering and anti-obesity(Reference Alappat and Awad15,Reference Shao, Yu and Chiang40–Reference Yang, Su and Yang42) . The serum lipid profiles were measured to explore effects of dietary CC on lipid metabolism of large yellow croaker. Reduced total cholesterol, TAG and LDL-cholesterol and increased HDL-cholesterol were observed in CC supplementation groups of this study, consistent with effects of CC on hypercholesterolaemia, hyperlipidaemia and non-alcoholic fatty liver(Reference Panahi, Kianpour and Mohtashami43–Reference Jang, Choi and Jung46). Collectively, lipid-lowering effects of CC on large yellow croaker might be shown in serum lipid profiles and lipid content of body and liver.
To further investigate how CC regulated lipid metabolism, expression of genes related to lipid metabolism in the liver was detected. PPARα has been shown to play a crucial role in reduced lipid accumulation via increasing oxidation and lipolysis of fatty acid(Reference Guzmán, Verme and Fu47–Reference Yoon49). The activation of PPARα up-regulates expression of aco and cpt1, which are involved in oxidation of fatty acids(Reference Mandard, Müller and Kersten50,Reference Kersten51) . In this study, mRNA expression of pparα, cpt1 and aco significantly up-regulated in CC supplementation groups, suggesting that CC might increase oxidation of fatty acids. Similar results were found in mammals that CC promotes expression of pparα and further activates cpt1 and aco (Reference Shin, Ha and McGregor13,Reference Um, Hwang and Ahn52) . SREBP positively participates in synthesising fatty acids, activating SREBP and then triggering FAS which is a critical lipogenic enzyme catalysing terminal steps in the de novo biogenesis of fatty acids(Reference Shin, Ha and McGregor13,Reference You, Fischer and Deeg53–Reference Horton, Goldstein and Brown55) . In the present study, CC reduced expression of srebp1 and fas, implying that de novo synthesis of fatty acids may be inhibited. Overall, CC reduced hepatic lipid deposition via promoting oxidation of fatty acids and decreasing de novo synthesis of fatty acids.
In addition to the lipid-lowering effects of CC, this study also found that CC could regulate the synthesis of PUFA in large yellow croaker. Dietary CC increased the ratio of n-6 PUFA:n-3 PUFA, while decreased SFA in the liver of large yellow croaker. These changes were probably due to improved SFA lipolysis(56). Reduced SFA might result in increased n-3 PUFA and n-6 PUFA in the liver. In addition, CC might promote the synthesis of PUFA. In this study, CC could up-regulate expression of elovl4, elovl5 and Δ6fad in the liver, which are speed limiting enzymes in the synthesis of PUFA(Reference Morais, Castanheira and Martinez-Rubio57,Reference Tocher58) . This function of CC was firstly found in fish. In the muscle, n-3 PUFA and n-6 PUFA were dose-dependently increased by dietary CC and there were no statistical differences among treatment groups. However, it is expected that PUFA of the muscle would be remarkably increased with the farming period extending. This finding is important because the fish muscle is an important source of PUFA for human beings.
The effects of dietary CC on alleviating liver damage and improving antioxidant capability also were investigated. aspartate transaminase and alanine transaminase are released from hepatocytes into the blood during liver injury; therefore, their activities are biomarkers of liver damage(Reference Kwo, Cohen and Lim59). Reduced serum aspartate transaminase and alanine transaminase was observed in this study, indicating that CC was able to alleviate liver damage induced by HFD. Its protective effects on the liver have also been found on other fish(Reference Manju, Akbarsha and Oommen38,Reference Cao, Ding and Du60,Reference Mahfouz61) . In addition, previous studies have confirmed that CC could improve antioxidant capacity in mammals(Reference Aggarwal and Harikumar11,Reference Wu, Lin and Chu62,Reference Menon and Sudheer63) . In this study, MDA (the biomarker of lipid peroxidation) levels were significantly decreased in CC supplementation groups, similar to results that dietary CC supplementation inhibited lipid peroxidation, and reduced MDA content in climbing perch(Reference Manju, Akbarsha and Oommen38), rainbow trout(Reference Akdemir, Orhan and Tuzcu64), crucian carps(Reference Jiang, Wu and Zhou21) and nile tilapia(Reference Mahmoud, Al-Sagheer and Reda20). Furthermore, this study also found that dietary CC increased total antioxidant capacity and the activity of SOD and CAT, which are crucial parameters in the antioxidant system. Similar results were found in climbing perch and rainbow trout that dietary CC increased activity of SOD and/or CAT(Reference Mahmoud, Al-Sagheer and Reda20,Reference Manju, Sherin and Rajasekharan37) . Collectively, these indicated that CC supplementation was likely to improve the health and function of the piscine liver through alleviating liver damage and improving antioxidant capacity. In addition, the increased n-6 PUFA and n-3 PUFA of the liver might be beneficial to the health of fish(Reference Zuo, Ai and Mai27,Reference Zuo, Ai and Mai65) . Therefore, the lower liver damage and higher antioxidant capacity might promote growth performance of large yellow croaker.
In conclusion, the present study showed that 0·04 % CC supplementation could mitigate the adverse effects of HFD and promote growth of large yellow croaker. The positive performance of CC might attribute to its role in reducing liver lipid deposition, improving the antioxidant activity and increasing the PUFA contents. Reduced abnormal hepatic lipid deposition was probably due to increased fatty acid oxidation and decreased de novo synthesis of fatty acids.
Acknowledgements
This research was supported by the National Science Fund for Distinguished Young Scholars of China (grant no. 31525024), Agriculture Research System of China (grant no. CARS-47-11) and Ten-thousand Talents Program (grant no. 2018-29).
Authors’ contributions were as follows: R. J.: conceptualisation, methodology, software, validation, formal analysis, investigation, writing the original draft, review and editing of the manuscript; X. X. and X. L.: investigation, validation, and writing the original draft; K. M.: data curation, resources and supervision; Q. A.: conceptualisation, data curation, resources, review and editing of the manuscript, supervision, project administration and funding acquisition.
There are no conflicts of interest to report.
Supplementary material
To view supplementary material for this article, please visit https://dx.doi.org/10.1017/S0007114520004171












