Abstract
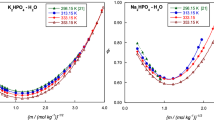
Densities of sodium hypophosphite aqueous solution (NaH2PO2) with the molality varied from 1.019143 to 10.43887 mol kg–1 at temperature intervals of 5 K range from 283.15 to 363.15 K at 101.325 kPa were measured by a precise Anton Paar Digital vibrating-tube densimeter. From the density data, the thermal expansion coefficients, apparent volume and partial molar volumes were obtained. According to the Pitzer ion-interaction equation of the apparent molar volumes, the Pitzer single-salt parameters and their temperature-dependent correlation for NaH2PO2 were firstly obtained by the least-squares method. The model shown that apparent molar volumes agree well with the experimental values, which indicated the single salt parameters and the temperature-dependent formula are reliable.



Similar content being viewed by others
REFERENCES
S. G. Maged., J. Mol. Catal. A: Chem. 248, 175 (2006). https://doi.org/10.1016/j.molcata.2005.12.014
D. Ma, J. Zhao, R. Chu, et al., Adv. Powder Technol. 24, 79 (2013). https://doi.org/10.1016/j.apt.2012.02.004
X. Qiu, Z. Li, X. Li, et al., Chem. Eng. J. 334, 108 (2018). https://doi.org/10.1016/j.cej.2017.09.194
F. M. Lessan, M. Montazer, and B. Moghadam, Thermochim. Acta 520, 48 (2011). https://doi.org/10.1016/j.tca.2011.03.012
C. Yujiao, Y. Gang, H. Bonian, et al., Powder Technol. 25, 477 (2014). https://doi.org/10.1016/j.apt.2013.07.003
X. Gan, K. Zhou, W. Hu, et al., Surf. Coat. Technol. 206, 3405 (2012). https://doi.org/10.1016/j.surfcoat.2012.02.006
E. Ravi and E. P. Brian, Mater. Lett. 76, 36 (2012). https://doi.org/10.1016/j.matlet.2012.02.049
A. Nazari, M. Montazer, A. Rashidi, et al., Appl. Catal., A: Gen. 371, 10 (2009). https://doi.org/10.1016/j.apcata.2009.08.029
Y. Xian, X. Guo, X. Hou, et al., J. Chromatogr. A 1526, 31 (2017). https://doi.org/10.1016/j.chroma.2017.10.047
S. Hashemikia and M. Montazer, Appl. Catal., A: Gen. 417–418, 200 (2012). https://doi.org/10.1016/j.apcata.2011.12.041
C. S. Gong. Technology and Application of Advanced Phosphorus. Chemical Engineering (Chemical Industry Press, Beijing, 2013).
L. Tovazhnyansky, P. Kapustenko, L. Ulyiev, et al., Appl. Therm. Eng. 30, 2306 (2010). https://doi.org/10.1016/j.applthermaleng.2010.04.021
N. Pittayagorn and D. Chanaiporn, Spectrochim. Acta, Part A 77, 890 (2010). https://doi.org/10.1016/j.saa.2010.08.028
V. Alisoglu and H. Necefoglu, C. R. Acad. Sci. Paris. Ser. IIb, 324, 139 (1997). https://doi.org/10.1016/S1251-8069(99)80017-7
V. Adiguzel, H. Erge, V. Alisoglu, et al., J. Chem. Thermodyn. 75, 35 (2014). https://doi.org/10.1016/j.jct.2014.04.014
J. R. Van Vazer, Encyclopedia of Chemical Technology (Interscience, New York, 1953).
W. E. Estes, US Patent 4521391 (1985).
P. Seferlis, J. Klemes, I. Bulatov, et al., Proceedings of the 9th Conference on Process Integration, Modelling and Optimization for Energy Saving and Pollution Reduction-PRES2006/CHISA (Prague, 2006), Vol. 4 [Open Access]
A. I. Papadopoulosa and P. Seferlisa, Chem. Eng. Process. 48, 493 (2009). https://doi.org/10.1016/j.cep.2008.06.011
M. M. Monica, M. M. Ivey, M.E. Lee, et al., J. Chromatogr. A 1039, 105 (2004). https://doi.org/10.1016/j.chroma.2003.11.056
The Chemical Industry Standard of China HG/T3253: Sodium Hypophosphite (2000).
K. Sun, P. Li, L. Li, et al., J. Chem. Thermodynamics 140, 105895 (2020). https://doi.org/10.1016/j.jct.2019.105895
J. A. Dean, Lange’s Handbook of Chemistry (Science, Beijing, 1991).
R. K. Ameta, M. Singh, R. K. Kale, et al., J. Chem. Thermodyn. 60, 159 (2013). https://doi.org/10.1016/j.jct.2013.01.012
W. G. Xu, Y. Qin, F. Gao, et al., J. Ind. Eng. Chem. Res. 53, 7217-7223 (2014). https://doi.org/10.1021/ie402040h
S. K. Lomesh, V. Nathan, M. Bala, and P. Thakur, J. Mol. Liq. 284, 241 (2019). https://doi.org/10.1016/j.molliq.2019.04.006
S. Mondal, S. S. Dhondge, L. J. Pailwal, et al., J. Chem. Thermodyn. 90, 147 (2015). https://doi.org/10.1016/j.jct.2015.06.025
H. W. Gea, H. J. Yanga, J. I Lia, et al., Russ. J. Inorg. Chem. 65, 222 (2020). https://doi.org/10.1134/S0036023620020059
H. Bin, H. Lubomir, and H. Glenn, J. Chem. Eng. Data 61, 3618 (2016). https://doi.org/10.1021/acs.jced.6b00519.
Y. Marcus and H.T. Glenn, Chem. Rev. 106, 4585 (2006). https://doi.org/10.1021/cr040087x
C. Akilan, H. T. Glenn, N. Rohman, et al., J. Phys. Chem. B 110, 14961 (2006). https://doi.org/10.1021/jp0620769
S. Rahmat and P. Hana, J. Chem. Thermodyn. 265, 173 (2008). https://doi.org/10.1016/j.fluid.2008.01.004
H. R. Rafiee and F. Frouzesh, J. Chem. Thermodyn. 102 (2016) 95. https://doi.org/10.1016/j.jct.2016.07.003
S. Rahmat and P. Hana, J. Chem. Thermodyn. 40, 1012 (2008). https://doi.org/10.1016/j.jct.2008.01.018
H. Bin, H. Lubomir, L. Wu, and H. Glenn, J. Chem. Eng. Data. 61, 1388 (2016). https://doi.org/10.1021/acs.jced.5b00535
K. S. Pitzer, J. Phys. Chem. 77, 268 (1973) [Open Access].
Z. Denis, D. Thomas, and S.V. Carmen, J. Chem. Eng. Data 60, 1181 (2015). https://doi.org/10.1021/je501152a
D. P. Fernandez, A. R. H. Goodwin, E. W. Lemmon, et al., J. Phys. Chem. Ref. Data 26, 1125−1166 (1997). https://doi.org/10.1063/1.555997
ACKNOWLEDGMENTS
Financial supports from the National Natural Science of China (U1607123 and 21773170), the Key Projects of Natural Science Foundation of Tianjin (18JCZDJC10040) and the Yangtze Scholars and Innovative Research Team in Chinese University (IRT_17R81) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no competing financial interest.
Supplementary material
Rights and permissions
About this article
Cite this article
Umarbek Alimov, Zhao, K., Guo, Y. et al. Volumetric Properties and Ion Interactions for Sodium Hypophosphite Aqueous Solution from 283.15 to 363.15 K at 101.325 kPa. Russ. J. Inorg. Chem. 65, 1913–1921 (2020). https://doi.org/10.1134/S0036023620120025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620120025