Abstract
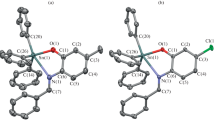
Two synthetic approaches were developed for the preparation of 3,6-di-tert-butyl-2-hydroxy-4-pyridinylphenolate (LH), a new zwitter-ionic redox-active diolate type ligand. Two heteroligand five-coordinate tin(IV) derivatives were prepared with this ligand: 3,6-di-tert-butyl-2-oxy-4-pyridinylphenolato(triphenyl)tin(IV) (I) and 3,6-di-tert-butyl-2-oxy-4-pyridinylphenolato(diphenyl)chlorotin(IV) (II). The molecular structures of the ligand LH ∙ 0.5Py and complex I ∙ CH3CN were determined by X-ray diffraction (CIF files CCDC nos. 1974166 (LH), 1974165 (I)). It was shown that the ligand LH and the tin(IV) compound exhibit solvatochromism, which consists in a considerable blue shift with increasing solvent polarity.







Similar content being viewed by others
REFERENCES
Abakumov, G.A., Piskunov, A.V., Cherkasov, V.K., et al., Russ. Chem. Rev., 2018, vol. 87, no. 5, p. 393.
Chegerev, M.G. and Piskunov, A.V., Russ. J. Coord. Chem., 2018, vol. 44, p. 258. https://doi.org/10.1134/S1070328418040036
Ershova, I.V. and Piskunov, A.V., Russ. J. Coord. Chem., 2020, vol. 46, p. 154. https://doi.org/10.1134/S1070328420030021
Poddel’sky, A.I., Cherkasov, V.K., and Abakumov, G.A., Coord. Chem. Rev., 2009, vol. 253, p. 291.
Tezgerevska, T., Alley, K.G., and Boskovic, C., Coord. Chem. Rev., 2014, vol. 268, p. 23.
Miller, J.S. and Min, K.S., Angew. Chem., Int. Ed. Engl., 2009, vol. 48, no. 2, p. 262.
Broere, D.L.J., Plessius, R., and van der Vlugt, J.I., Chem. Soc. Rev., 2015, vol. 44, p. 6886.
Dzik, W.I., van der Vlugt, J.I., Reek, J.N.H., and de Bruin, B., Angew. Chem., Int. Ed. Engl., 2011, vol. 50, p. 3356.
Luca, O.R. and Crabtree, R.H., Chem. Soc. Rev., 2013, vol. 42, p. 1440.
Lyaskovskyy, V. and de Bruin, B., ACS Catal., 2012, vol. 2, p. 270.
Fedushkin, I.L., Nikipelov, A.S., Morozov, A.G., et al., Chem.-Eur. J., 2012, vol. 18, p. 255.
Abakumov, G.A., Cherkasov, V.K., Piskunov, A.V., et al., Dokl. Chem., 2009, vol. 427, no. 1, p. 168.
Piskunov, A.V., Meshcheryakova, I.N., Fukin, G.K., et al., Russ. J. Coord. Chem, 2014, vol. 40, no. 4, p. 205. https://doi.org/10.1134/S1070328414040083
Piskunov, A.V., Meshcheryakova, I.N., Fukin, G.K., et al., Russ. J. Coord. Chem, 2017, vol. 43, no. 12, p. 816. https://doi.org/10.1134/S1070328417120077
Koshechkov, K.A., Zemlyanskii, N.N., Sheverdina, N.I., and Panov, E.M., Metody elementoorganicheskoi khimii. Germanii, olovo, svinets (Methods of Organoelement Chemistry. Germanium. Tin. Lead), Moscow: Nauka, 1968.
Morris, A.M., Pierpont, C.G., and Finke, R.G., Inorg. Chem., 2009, vol. 48, p. 3496.
Meshcheryakova, I.N., Shavyrin, A.S., Cherkasov, A.V., and Piskunov, A.V., Russ. Chem. Bull., 2019, vol. 68, no. 7, p. 1414.
Garnov, V.A., Nevodchikov, V.I., Abakumov, G.A., et al., Russ. Chem. Bull., 1985, p. 2589.
Gordon, A.J. and Ford, R.A., The Chemist’s Companion, New York: Wiley, 1972.
Smart APEX2, Madison: Bruker AXS Inc., 2014.
Krause, L., Herbst-Irmer, R., Sheldrick, G.M., and Stalke, D., J. Appl. Crystallogr., 2015, vol. 48, p. 3.
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, p. 3.
Shurygina, M.P., Druzhkov, N.O., Arsen’ev M.V., et al., Russ. J. Org. Chem., 2011, vol. 47, p. 486.
Abakumov, G.A., Cherkasov, V.K., Kocherova, T.N., et al., Russ. Chem. Bull., 2006, vol. 55, p. 1195.
Druzhkov, N.O., Meshcheryakova, I.N., Cherkasov, A.V., and Piskunov, A.V., Russ. Chem. Bull., 2020, vol. 69, no. 1, p. 49.
Bakewell, N., Thavarajah, R., Motevalli, M., and Sheriff, T.S., New J. Chem., 2017, vol. 41, p. 15411.
Panja, A. and Frontera, A., Eur. J. Inorg. Chem., 2018, vol. 7, p. 924.
Panja, A., Jana, N.C., Patra, M., et al., J. Mol. Cat. A, 2016, vol. 412, p. 56.
Sheriff, T.S., Watkinson, M., Motevallia, M., and Lesin, J.F., Dalton Trans., 2010, vol. 39, p. 53.
Kuropatov, V.A., Cherkasov, V.K., Fukin, G.K., and Abakumov, G.A., Russ. Chem. Bull., 2011, vol. 60, p. 2291.
Fukin, G.K., Cherkasov, A.V., Shurygina, M.P., et al., Struct. Chem., 2010, vol. 21, p. 607.
Addison, A.W., Rao, T.N., Reedijk, J., et al., Dalton Trans., 1984, no. 7, p. 1349.
Batsanov, S.S., Zh. Neorg. Khim., 1991, vol. 36, p. 3015.
Brown, S.N., Inorg. Chem., 2012, vol. 51, p. 1251.
Reichhardt, C., Solvents and Solvent Effects in Organic Chemistry, Weinheim: VCH, 1988.
Snyder, L.R., J. Chromatogr., 1974, vol. 92, no. 2, p. 223.
ACKNOWLEDGMENTS
The studies were carried out using the research equipment of the Center for Collective Use “Analytical Center of the Razuvaev Institute of Organometallic Chemistry” at the Razuvaev Institute of Organometallic Chemistry, Russian Academy of Sciences, and was supported by the Federal Target Program “Research and Development along the Priority Trends of the Science and Technology Sector of Russia for 2014–2020” (project unique identifier: RFMEFI62120X0040).
Funding
The study was supported by the Russian Foundation for Basic Research (project no. 19-29-08039-mk).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicst of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Piskunov, A.V., Pashanova, K.I., Mart’yanov, K.A. et al. 3,6-Di-tert-Butyl-2-Hydroxy-4-Pyridinylphenolate and Tin(IV) Complexes it Forms: Synthesis and Structure Details and Solvatochromic Effect. Russ J Coord Chem 46, 795–804 (2020). https://doi.org/10.1134/S1070328420120064
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328420120064