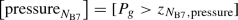Abstract
Animals exhibit remarkable feats of behavioral flexibility and multifunctional control that remain challenging for robotic systems. The neural and morphological basis of multifunctionality in animals can provide a source of bioinspiration for robotic controllers. However, many existing approaches to modeling biological neural networks rely on computationally expensive models and tend to focus solely on the nervous system, often neglecting the biomechanics of the periphery. As a consequence, while these models are excellent tools for neuroscience, they fail to predict functional behavior in real time, which is a critical capability for robotic control. To meet the need for real-time multifunctional control, we have developed a hybrid Boolean model framework capable of modeling neural bursting activity and simple biomechanics at speeds faster than real time. Using this approach, we present a multifunctional model of Aplysia californica feeding that qualitatively reproduces three key feeding behaviors (biting, swallowing, and rejection), demonstrates behavioral switching in response to external sensory cues, and incorporates both known neural connectivity and a simple bioinspired mechanical model of the feeding apparatus. We demonstrate that the model can be used for formulating testable hypotheses and discuss the implications of this approach for robotic control and neuroscience.












Similar content being viewed by others
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00422-021-00865-x
References
Aggarwal S, Chugh N (2019) Signal processing techniques for motor imagery brain computer interface: a review. Array 1–2(January):100003. https://doi.org/10.1016/j.array.2019.100003
Ayers J (1995) A reactive ambulatory robot architecture for operation in current and surge. In: Autonomous vehicles in mine countermeasures symposium, April 1995, pp 1–14. http://www.neurotechnology.neu.edu/nps95mcmmanuscript.html
Ayers J (2002) A conservative biomimetic control architecture for autonomous underwater robots. Neurotechnol Biomimetic Robots. https://doi.org/10.7551/mitpress/4962.003.0019
Ayers JL, Davis WJ (1977) Neuronal control of locomotion in the lobster Homarus americanus. J Comp Physiol A 115(1):29–46. https://doi.org/10.1007/bf00667783
Bässler U (1988) Functional principles of pattern generation for walking movements of stick insect forelegs: the role of the femoral chordotonal organ afferences. J Exp Biol 136(1):125–147
Bazenkov NI, Boldyshev BA, Dyakonova V, Kuznetsov OP (2020) Simulating small neural circuits with a discrete computational model. Biol Cybern. https://doi.org/10.1007/s00422-020-00826-w
Beck JM, Pouget A (2007) Exact inferences in a neural implementation of a hidden Markov model. Neural Comput 19(5):1344–1361. https://doi.org/10.1162/neco.2007.19.5.1344
Beer RD, Chiel HJ (1999) Gallagher JC Evolution and analysis of model CPGs for walking: II. General principles and individual variability. J Comput Neurosci 7(2):119–147. https://doi.org/10.1023/A:1008920021246
Beer RD, Chiel HJ, Quinn RD, Espenschied KS (1992) A distributed neural network architecture for hexapod robot locomotion. Neural Comput 4(3):356–365
Beer RD, Chiel HJ, Sterling LS (1990) A biological perspective on autonomous agent design. Robot Autonom Syst 6(1):169–186. https://doi.org/10.1016/S0921-8890(05)80034-X
Bicanski A, Ryczko D, Knuesel J, Harischandra N, Charrier V, Ekeberg Ö, Cabelguen JM, Ijspeert AJ (2013) Decoding the mechanisms of gait generation in salamanders by combining neurobiology, modeling and robotics. Biol Cybern 107(5):545–564. https://doi.org/10.1007/s00422-012-0543-1
Bidaye SS, Laturney M, Chang AK, Liu Y, Bockemühl T, Büschges A, Scott K (2020) Two brain pathways initiate distinct forward walking programs in drosophila. Neuron. https://doi.org/10.1016/j.neuron.2020.07.032
Blümel M, Guschlbauer C, Daun-Gruhn S, Hooper SL, Büschges A (2012) Hill-type muscle model parameters determined from experiments on single muscles show large animal-to-animal variation. Biol Cybern 106(10):559–571
Blümel M, Guschlbauer C, Hooper SL, Büschges A (2012) Using individual-muscle specific instead of across-muscle mean data halves muscle simulation error. Biol Cybern 106(10):573–585
Blümel M, Hooper SL, Guschlbauerc C, White WE, Büschges A (2012) Determining all parameters necessary to build Hill-type muscle models from experiments on single muscles. Biol Cybern 106(10):543–558
Brosch T, Neumann H (2014) Interaction of feedforward and feedback streams in visual cortex in a firing-rate model of columnar computations. Neural Netw 54:11–16. https://doi.org/10.1016/j.neunet.2014.02.005
Brown JW, Caetano-Anollés D, Catanho M, Gribkova E, Ryckman N, Tian K, Voloshin M, Gillette R (2018) Implementing goal-directed foraging decisions of a simpler nervous system in simulation. eNeuro 5(1):1–10. https://doi.org/10.1523/ENEURO.0400-17.2018
Büschges A (2005) Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol 93(3):1127–1135. https://doi.org/10.1152/jn.00615.2004
Büschges A, Akay T, Gabriel JP, Schmidt J (2008) Organizing network action for locomotion: insights from studying insect walking. Brain Res Rev 57(1):162–171
Cappellini G, Ivanenko YP, Poppele RE, Lacquaniti F (2006) Motor patterns in human walking and running. J Neurophysiol 95(6):3426–3437. https://doi.org/10.1152/jn.00081.2006
Cash D, Carew TJ (1989) A quantitative analysis of the development of the central nervous system in juvenile Aplysia californica. J Neurobiol 20(1):25–47. https://doi.org/10.1002/neu.480200104
Cataldo E, Byrne JH, Baxter DA (2006) Computational model of a central pattern generator. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics) 4210 LNBI, 242–256. https://doi.org/10.1007/11885191_17
Chiel HJ, Beer RD (1997) The brain has a body: adaptive behavior emerges from interactions of nervous system, body and environment. Trends Neurosci 20(12):553–557. https://doi.org/10.1016/S0166-2236(97)01149-1
Chiel HJ, Beer RD, Gallagher JC (1999) Evolution and analysis of model CPGs for walking: I. Dynamical modules. J Comput Neurosci 7(2):99–118. https://doi.org/10.1023/A:1008920021246
Chiel HJ, Crago P, Mansour JM, Hathi K (1992) Biomechanics of a muscular hydrostat: a model of lapping by a reptilian tongue. Biol Cybern 67(5):403–415. https://doi.org/10.1007/BF00200984
Chiel HJ, Kupfermann I, Weiss KR (1988) An identified histaminergic neuron can modulate the outputs of buccal–cerebral interneurons in Aplysia via presynaptic inhibition. J Neurosci 8(January):49–63
Chiel HJ, Ting LH, Ekeberg Ö, Hartmann MJ (2009) The brain in its body: motor control and sensing in a biomechanical context. J Neurosci 29(41):12807–12814. https://doi.org/10.1523/JNEUROSCI.3338-09.2009
Chiel HJ, Weiss KR, Kupfermann I (1986) An identified histaminergic neuron modulates feeding motor circuitry in Aplysia. J Neurosci 6(8):2427–2450. https://doi.org/10.1523/jneurosci.06-08-02427.1986
Church PJ, Lloyd PE (1994) Activity of multiple identified motor neurons recorded intracellularly during evoked feeding-like motor programs in Aplysia. J Neurophysiol 72(4):1794–1809. https://doi.org/10.1152/jn.1994.72.4.1794
Church PJ, Whim MD, Lloyd PE (1993) Modulation of neuromuscular transmission by conventional and peptide transmitters released from excitatory and inhibitory motor neurons in Aplysia. J Neurosci 13(7):2790–2800. https://doi.org/10.1523/jneurosci.13-07-02790.1993
Connor JA, Kretz R, Shapiro E (1986) Calcium levels measured in a presynaptic neurone of Aplysia under conditions that modulate transmitter release. J Physiol 375(1):625–642. https://doi.org/10.1113/jphysiol.1986.sp016137
Costa RM, Baxter DA, Byrne JH (2020) Computational model of the distributed representation of operant reward memory: combinatoric engagement of intrinsic and synaptic plasticity mechanisms. Learn Memory 27:236–249. https://doi.org/10.1101/lm.051367.120
Cropper EC, Jing J, Weiss KR (2019) The feeding network of Aplysia. In: The Oxford handbook of invertebrate neurobiology, December. Oxford University Press, pp 400–422. https://doi.org/10.1093/oxfordhb/9780190456757.013.19
Cullins MJ, Chiel HJ (2010) Electrode fabrication and implantation in Aplysia californica for multi-channel neural and muscular recordings in intact, freely behaving animals. J Vis Exp 40:e1791. https://doi.org/10.3791/1791
Cullins MJ, Gill JP, McManus JM, Lu H, Shaw KM, Chiel HJ (2015) Sensory feedback reduces individuality by increasing variability within subjects. Curr Biol 25(20):2672–2676. https://doi.org/10.1016/j.cub.2015.08.044
Cullins MJ, Shaw KM, Gill JP, Chiel HJ (2015) Motor neuronal activity varies least among individuals when it matters most for behavior. J Neurophysiol 113(3):981–1000. https://doi.org/10.1152/jn.00729.2014
Dallidis SE, Karafyllidis IG (2014) Boolean network model of the Pseudomonas aeruginosa quorum sensing circuits. IEEE Trans Nanobiosci 13(3):343–349. https://doi.org/10.1109/TNB.2014.2345439
Danner SM, Wilshin SD, Shevtsova NA, Rybak IA (2016) Central control of interlimb coordination and speed-dependent gait expression in quadrupeds. J Physiol 594(23):6947–6967. https://doi.org/10.1113/JP272787
De Jong H (2002) Modeling and simulation of genetic regulatory systems: a literature review. J Comput Biol 9(1):67–103. https://doi.org/10.1089/10665270252833208
Destexhe A, Sejnowski TJ (2009) The Wilson–Cowan model, 36 years later. Biol Cybern 101(1):1–2. https://doi.org/10.1007/s00422-009-0328-3
Drushel RF, Neustadter DM, Hurwitz I, Crago PE, Chiel HJ (1998) Kinematic models of the buccal mass of Aplysia californica. J Exp Biol 201(Pt 10):1563–83
Edwards R, Siegelmann HT, Aziza K, Glass L (2001) Symbolic dynamics and computation in model gene networks. Chaos 11(1):160–169. https://doi.org/10.1063/1.1336498
Eisenberg E, Hill TL, Chen Y (1980) Cross-bridge model of muscle contraction. Quantitative analysis. Biophys J 29(2):195–227. https://doi.org/10.1016/S0006-3495(80)85126-5
Ekeberg Ö (1993) A combined neuronal and mechanical model of fish swimming. Biol Cybern 69(5–6):363–374. https://doi.org/10.1007/bf00199436
Ekeberg Ö, Wallén P, Lansner A, Tråvén H, Brodin L, Grillner S (1991) A computer based model for realistic simulations of neural networks. Biol Cybern 65(2):81–90. https://doi.org/10.1007/bf00202382
Ermentrout B (2010) Neural networks as spatio-temporal pattern-forming systems. Rep Prog Phys 61(1998):353–430
Evans CG, Cropper EC (1998) Proprioceptive input to feeding motor programs in Aplysia. J Neurosci 18(19):8016–8031. https://doi.org/10.1523/jneurosci.18-19-08016.1998
Feng K, Sen R, Minegishi R, Dübbert M, Bockemühl T, Büschges A, Dickson BJ (2020)Distributed control of motor circuits for backward walking in drosophila. bioRxiv. https://doi.org/10.1101/2020.07.11.198663. https://www.biorxiv.org/content/early/2020/07/12/2020.07.11.198663
Gardner D (1977) Interconnections of identified multiaction interneurons in buccal ganglia of Aplysia. J Neurophysiol 40(2):349–361. https://doi.org/10.1152/jn.1977.40.2.349
Georgopoulos AP, Ashe J, Smyrnis N, Taira M (1992) The motor cortex and the coding of force. Science 256(5064):1692–1695. https://doi.org/10.1126/science.256.5064.1692
Georgopoulos AP, Kettner RE, Schwartz AB (1988) Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci 8(8):2928–2937. https://doi.org/10.1523/jneurosci.08-08-02928.1988
Giacomantonio CE, Goodhill GJ (2010) A Boolean model of the gene regulatory network underlying mammalian cortical area development. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1000936
Gill JP, Chiel HJ (2020) Rapid adaptation to changing mechanical load by ordered recruitment of identified motor neurons. eNeuro. https://doi.org/10.1523/ENEURO.0016-20.2020
Gill JP, Garcia S, Ting LH, Wu M, Chiel HJ (2020) neurotic: neuroscience tool for interactive characterization. eNeuro. https://doi.org/10.1523/ENEURO.0085-20.2020
Gill JP, Vorster APA, Lyttle DN, Keller TA, Stork SC, Chiel HJ (2018) Neural correlates of adaptive responses to changing load in feeding Aplysia. Poster presented at Society for Neuroscience 48th Annual Meeting, San Diego, CA. https://www.abstractsonline.com/pp8/#!/4649/presentation/17445
Glaser JI, Chowdhury RH, Perich MG, Miller LE, Kording KP (2017) Machine learning for neural decoding. arXiv:1708.00909
Golowasch J, Goldman MS, Abbott LF, Marder E (2002) Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol 87(2):1129–1131. https://doi.org/10.1152/jn.00412.2001
Harischandra N, Cabelguen JM, Ekeberg Ö (2010) A 3D musculo-mechanical model of the salamander for the study of different gaits and modes of locomotion. Front Neurorobotics 4(DEC):1–10. https://doi.org/10.3389/fnbot.2010.00112
Harris SE, Sawhill BK, Wuensche A, Kauffman S (2002) A model of transcriptional regulatory networks based on biases in the observed regulation rules. Complexity 7(4):23–40. https://doi.org/10.1002/cplx.10022
Haselgrove JC, Huxley HE (1973) X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. https://doi.org/10.1016/0022-2836(73)90222-2
Hausknecht M, Stone P (2015) Deep recurrent q-learning for partially observable MDPS. AAAI Fall Symposium—Technical Report, FS-15-06, pp 29–37
Heuer H, Schmidt RA, Ghodsian D (1995) Generalized motor programs for rapid bimanual tasks: a two-level multiplicative-rate model. Biol Cybern 73(4):343–356. https://doi.org/10.1007/BF00199470
Hill A (1938) The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond Ser B Biol Sci 126(843):136–195. https://doi.org/10.1098/rspb.1938.0050
Hodgkin A, Huxley A (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117(4):500
Hooper SL, Guschlbauer C, von Uckermann G, Büschges A (2006) Natural neural output that produces highly variable locomotory movements. J Neurophysiol 96(4):2072–2088. https://doi.org/10.1152/jn.00366.2006 PMID: 16775206
Hooper SL, Guschlbauer C, von Uckermann G, Büschges A (2007) Different motor neuron spike patterns produce contractions with very similar rises in graded slow muscles. J Neurophysiol 97(2):1428–1444. https://doi.org/10.1152/jn.01014.2006
Horchler AD, Daltorio KA, Chiel HJ, Quinn RD (2015) Designing responsive pattern generators: stable heteroclinic channel cycles for modeling and control. Bioinspir Biomimetics 10(2):026001
Hosman T, Vilela M, Milstein D, Kelemen JN, Brandman DM, Hochberg LR, Simeral JD (2019) BCI decoder performance comparison of an LSTM recurrent neural network and a Kalman filter in retrospective simulation. In: International IEEE/EMBS conference on neural engineering, NER 2019-March, pp 1066–1071. https://doi.org/10.1109/NER.2019.8717140
Huang Z, Satterlie RA (1990) Neuronal mechanisms underlying behavioral switching in a Pteropod mollusc. J Comp Physiol A 166(6):875–887. https://doi.org/10.1007/BF00187335
Hunt A, Schmidt M, Fischer M, Quinn R (2015) A biologically based neural system coordinates the joints and legs of a tetrapod. Bioinspir Biomimetics. https://doi.org/10.1088/1748-3190/10/5/055004
Hunt A, Szczecinski N, Quinn R (2017) Development and training of a neural controller for hind leg walking in a dog robot. Front Neurorobotics 11(APR):1–16. https://doi.org/10.3389/fnbot.2017.00018
Hurwitz I, Goldstein RS, Susswein AJ (1994) Compartmentalization of pattern-initiation and motor functions in the b31 and b32 neurons of the buccal ganglia of Aplysia californica. J Neurophysiol 71(4):1514–27. https://doi.org/10.1152/jn.1994.71.4.1514
Hurwitz I, Susswein AJ (1992) Adaptation of feeding sequences in Aplysia oculifera to changes in the load and width of food. J Exp Biol 166(1):215–235
Hurwitz I, Susswein AJ (1996) B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol 75(4):1327–1344. https://doi.org/10.1152/jn.1996.75.4.1327
Ivashko DG, Prilutsky BI, Markin SN, Chapin JK, Rybak IA (2003) Modeling the spinal cord neural circuitry controlling cat hindlimb movement during locomotion. Neurocomputing 52:621–629
Izhikevich E (2003) Simple model of spiking neurons. IEEE Trans Neural Netw 14(6):1569–1572. https://doi.org/10.1109/TNN.2003.820440
Izhikevich EM (2000) Neural excitability, spiking and bursting. Int J Bifur Chaos 10(06):1171–1266. https://doi.org/10.1142/S0218127400000840
Jaques N, Gu S, Turner RE, Eck D (2017) Workshop track-ICLR 2017 tuning recurrent neural networks with re-inforcement learning. ICLR 2017:1–13
Jing J, Cropper EC, Hurwitz I, Weiss KR (2004) The construction of movement with behavior-specific and behavior-independent modules. J Neurosci 24(28):6315–6325. https://doi.org/10.1523/JNEUROSCI.0965-04.2004
Jing J, Cropper EC, Weiss KR (2017) Network functions of electrical coupling present in multiple and specific sites in behavior-generating circuits. Elsevier Inc., Amsterdam. https://doi.org/10.1016/B978-0-12-803471-2.00005-9
Jing J, Weiss KR (2001) Neural mechanisms of motor program switching in Aplysia. J Neurosci 21(18):7349–7362. https://doi.org/10.1523/jneurosci.21-18-07349.2001
Jing J, Weiss KR (2002) Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci 22(14):6228–6238. https://doi.org/10.1523/jneurosci.22-14-06228.2002
Jing J, Weiss KR (2005) Generation of variants of a motor act in a modular and hierarchical motor network. Curr Biol 15(19):1712–1721. https://doi.org/10.1016/j.cub.2005.08.051
Kabotyanski EA, Baxter DA, Byrne JH (1998) Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol 79(2):605–21. https://doi.org/10.1152/jn.1998.79.2.605
Kamali Sarvestani I, Kozlov A, Harischandra N, Grillner S, Ekeberg Ö (2013) A computational model of visually guided locomotion in lamprey. Biol Cybern 107(5):497–512. https://doi.org/10.1007/s00422-012-0524-4
Kandel E (1976) Cellular basis of behavior: an introduction to behavioral neurobiology. Books in psychology. W. H. Freeman, San Francisco
Katzoff A, Ben-Gedalya T, Hurwitz I, Miller N, Susswein YZ, Susswein AJ (2006) Nitric oxide signals that Aplysia have attempted to eat, a necessary component of memory formation after learning that food is inedible. J Neurophysiol 96(3):1247–1257. https://doi.org/10.1152/jn.00056.2006 PMID: 16738221
Kauffman SA (1993) The origins of order: self-organization and selection in evolution. Oxford University Press, Oxford
Koch C, Segev I et al (1998) Methods in neuronal modeling: from ions to networks. MIT Press, Cambridge
Koehl MA (2006) Wave-swept shore: the rigors of life on a rocky coast. University of California Press, California
Kuo AD (2002) The relative roles of feedforward and feedback in the control of rhythmic movements. Mot Control 6(2):129–145
Kupfermann I (1974) Feeding behavior in Aplysia: a simple system for the study of motivation. Behav Biol 10(1):1–26. https://doi.org/10.1016/S0091-6773(74)91644-7
Latash M (1999) Progress in motor control: Bernstein’s traditions in movement studies. J Athletic Training 34(3):1999
Li L, Van Den Bogert EC, Caldwell GE, Van Emmerik RE, Hamill J (1999) Coordination patterns of walking and running at similar speed and stride frequency. Hum Mov Sci 18(1):67–85. https://doi.org/10.1016/S0167-9457(98)00034-7
Lu CW, Patil PG, Chestek CA (2012) Chapter seven—current challenges to the clinical translation of brain machine interface technology. In: Hamani C, Moro E (eds) Emerging horizons in neuromodulation, International Review of Neurobiology, vol 107. Academic Press, New York, pp 137–160. https://doi.org/10.1016/B978-0-12-404706-8.00008-5
Lu H, McManus JM, Chiel HJ (2013) Extracellularly identifying motor neurons for a muscle motor pool in Aplysia californica. J Vis Exp 73:e50189. https://doi.org/10.3791/50189
Lyttle DN, Gill JP, Shaw KM, Thomas PJ, Chiel HJ (2017) Robustness, flexibility, and sensitivity in a multifunctional motor control model. Biol Cybern 111(1):25–47. https://doi.org/10.1007/s00422-016-0704-8
Mann RA, Hagy J (1980) Biomechanics of walking, running, and sprinting. Am J Sports Med 8(5):345–350. https://doi.org/10.1177/036354658000800510
Mantziaris C, Bockemühl T, Büschges A (2020) Central pattern generating networks in insect locomotion. Dev Neurobiol
Marder E, Taylor AL (2011) Multiple models to capture the variability in biological neurons and networks. Nat Neurosci 14(2):133–138
Markin SN, Klishko AN, Shevtsova NA, Lemay MA, Prilutsky BI, Rybak IA (2016) A neuromechanical model of spinal control of locomotion. In: Prilutsky, Boris I., Edwards, Donald H. (Eds.) Neuromechanical modeling of posture and locomotion. Springer, Berlin, pp 21–65
McCulloch WS, Pitts W (1943) A logical calculus of the ideas immanent in nervous activity. Bull Math Biophys 5(4):115–133. https://doi.org/10.1007/BF02478259
McManus JM, Lu H, Chiel HJ (2012) An in vitro preparation for eliciting and recording feeding motor programs with physiological movements in Aplysia californica. JoVE J Vis Exp 70:e4320
McManus JM, Lu H, Cullins MJ, Chiel HJ (2014) Differential activation of an identified motor neuron and neuromodulation provide Aplysia’s retractor muscle an additional function. J Neurophysiol 112(4):778–791
Mihalaş S, Niebur E (2009) A generalized linear integrate-and-fire neural model produces diverse spiking behaviors. Neural Comput 21(3):704–718. https://doi.org/10.1162/neco.2008.12-07-680
Molkov YI, Bacak BJ, Talpalar AE, Rybak IA (2015) Mechanisms of left-right coordination in mammalian locomotor pattern generation circuits: a mathematical modeling view. PLoS Comput Biol 11(5):e1004270
Morgan PT, Jing J, Vilim FS, Weiss KR (2002) Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol 87(1):49–61. https://doi.org/10.1152/jn.00438.2001
Moritani T, Oddsson L, Thorstensson A (1991) Phase-dependent preferential activation of the soleus and gastrocnemius muscles during hopping in humans. J Electromyogr Kinesiol 1(1):34–40. https://doi.org/10.1016/1050-6411(91)90024-Y
Morton D, Chiel H (1994) Neural architectures for adaptive behavior. Trends Neurosci 17(10):413–420. https://doi.org/10.1016/0166-2236(94)90015-9
Morton DW, Chiel HJ (1993) In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A Sens Neural Behav Physiol 172(1):17–32. https://doi.org/10.1007/bf00214712
Morton DW, Chiel HJ (1993) The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A 173(5):519–536. https://doi.org/10.1007/BF00197761
Mulgaonkar Y, Araki B, Koh JS, Guerrero-Bonilla L, Aukes DM, Makineni A, Tolley MT, Rus D, Wood RJ, Kumar V (2016) The flying monkey: A mesoscale robot that can run, fly, and grasp. In: Proceedings—IEEE international conference on robotics and automation, 2016-June, pp 4672–4679. https://doi.org/10.1109/ICRA.2016.7487667
Neustadter DM, Drushel RF, Crago PE, Adams BW, Chiel HJ (2002) A kinematic model of swallowing in Aplysia californica based on radula/odontophore kinematics and in vivo magnetic resonance images. J Exp Biol 205(20):3177–3206
Neustadter DM, Herman RL, Drushel RF, Chestek DW, Chiel HJ (2007) The kinematics of multifunctionality: comparisons of biting and swallowing in Aplysia californica. J Exp Biol 210(2):238–260
Nicolelis MA, Lebedev MA (2009) Principles of neural ensemble physiology underlying the operation of brain–machine interfaces. Nat Rev Neurosci 10(7):530–540. https://doi.org/10.1038/nrn2653
Novakovic VA, Sutton GP, Neustadter DM, Beer RD, Chiel HJ (2006) Mechanical reconfiguration mediates swallowing and rejection in Aplysia californica. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192(8):857–870. https://doi.org/10.1007/s00359-006-0124-7
Oishi K, Klavins E (2014) Framework for engineering finite state machines in gene regulatory networks. ACS Synth Biol 3(9):652–665. https://doi.org/10.1021/sb4001799
Packard N, Wolfram S (1985) Two-dimensional cellular automata. J Stat Phys 38(March):901–946. https://doi.org/10.1201/9780429494093-6
Payne JL, Wagner A (2013) Constraint and contingency in multifunctional gene regulatory circuits. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.1003071
Pearson K (1993) Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci 16(1):265–297
Pearson KG (1987) Central pattern generation: a concept under scrutiny. Springer, Boston, pp 167–185. https://doi.org/10.1007/978-1-4615-9492-5_10
Piazzesi G, Lombardi V (1995) A cross-bridge model that is able to explain mechanical and energetic properties of shortening muscle. Biophys J 68(5):1966–1979. https://doi.org/10.1016/S0006-3495(95)80374-7
Prescott TJ, Ayers JL, Grasso F, Verschure PFMJ (2016) Chapter 17. Embodied models and neurorobotics. In: Embodied M (ed) From neuron to cognition via computational neuroscience. MIT Press, Cambridge, pp 483–512
Prinz AA, Bucher D, Marder E (2004) Similar network activity from disparate circuit parameters. Nat Neurosci 7(12):1345–1352
Ravn AP, Rischel H, Holdgaard M, Eriksen TJ, Conrad F, Andersen TO (1995) Hybrid control of a robot—a case study. Hybrid Syst II:391–404. https://doi.org/10.1007/3-540-60472-3_20
Rivera Torres PJ, Serrano Mercado EI, Anido Rifón L (2018) Probabilistic Boolean network modeling of an industrial machine. J Intell Manuf 29(4):875–890. https://doi.org/10.1007/s10845-015-1143-4
Röschard J, Roces F (2003) Cutters, carriers and transport chains: distance-dependent foraging strategies in the grass-cutting ant Atta vollenweideri. Insectes Soc 50(3):237–244. https://doi.org/10.1007/s00040-003-0663-7
Rosin DP, Rontani D, Gauthier DJ, Schöll E (2013) Experiments on autonomous boolean networks. Chaos 23:2. https://doi.org/10.1063/1.4807481
Royakkers L, van Est R (2015) A literature review on new robotics: automation from love to war. Int J Soc Robot 7(5):549–570. https://doi.org/10.1007/s12369-015-0295-x
Saadatpour A, Albert I, Albert R (2010) Attractor analysis of asynchronous Boolean models of signal transduction networks. J Theor Biol 266(4):641–656. https://doi.org/10.1016/j.jtbi.2010.07.022
Kleene SC (1951) Representation of events in nerve nets and finite automata. Technical report, U.S. Air Force Project RAND
Schwartz AB, Kettner RE, Georgopoulos AP (1988) Primate motor cortex and free arm movements to visual targets in three-dimensional space. I. Relations between single cell discharge and direction of movement. J Neurosci 8(8):2913–2927. https://doi.org/10.1523/jneurosci.08-08-02913.1988
Selverston AI (1992) Dynamic biological networks: the stomatogastric nervous system. MIT Press, Cambridge
Selverston AI, Russell DF, Miller JP, King DG (1976) The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7:215–289. https://doi.org/10.1016/0301-0082(76)90008-3
Sewak M (2019) Deep reinforcement learning. In: Deep reinforcement learning, pp 1–9. https://doi.org/10.1007/978-981-13-8285-7
Shadmehr R (1970) A mathematical muscle model. ReCALL
Shaw KM, Lyttle DN, Gill JP, Cullins MJ, Mcmanus JM, Lu H, Thomas PJ, Chiel HJ (2015) The significance of dynamical architecture for adaptive responses to mechanical loads during rhythmic behavior. J Comput Neurosci 38:25–51. https://doi.org/10.1007/s10827-014-0519-3
Shea-Brown E, Rinzel J, Rakitin BC, Malapani C (2006) A firing rate model of Parkinsonian deficits in interval timing. Brain Res 1070(1):189–201. https://doi.org/10.1016/j.brainres.2005.10.070
Shev M, Strang G (2016) ”Operator splitting.” Splitting Methods in Communication, Imaging, Science, and Engineering. Springer 95–114
Shoham S, Paninski LM, Fellows MR, Hatsopoulos NG, Donoghue JP, Normann RA (2005) Statistical encoding model for a primary motor cortical brain–machine interface. IEEE Trans Biomed Eng 52(7):1312–1322. https://doi.org/10.1109/TBME.2005.847542
Siegle L, Schwab JD, Kühlwein SD, Lausser L, Tümpel S, Pfister AS, Kühl M, Kestler HA (2018) A boolean network of the crosstalk between IGF and wnt signaling in aging satellite cells. PLoS ONE 13(3):1–24. https://doi.org/10.1371/journal.pone.0195126
Stamhuis E, Aerts P, Nauwelaerts S (2005) Swimming and jumping in a semi-aquatic frog. Animal Biol 55(1):3–15
Stehouwer DJ (1992) Development of anuran locomotion: ethological and neurophysiological considerations. J Neurobiol 23(10):1467–1485. https://doi.org/10.1002/neu.480231008
Stewart HL (2004) Consequences of flexural stiffness and buoyancy for hydrodynamic forces, light interception and dispersal of a tropical alga. University of California, Berkeley
Sussillo D, Nuyujukian P, Fan JM, Kao JC, Stavisky SD, Ryu S, Shenoy K (2012) A recurrent neural network for closed-loop intracortical brain-machine interface decoders. J Neural Eng. https://doi.org/10.1088/1741-2560/9/2/026027
Susswein AJ, Byrne JH (1988) Identification and characterization of neurons initiating patterned neural activity in the buccal ganglia of Aplysia. J Neurosci 8(6):2049–2061
Susswein AJ, Chiel HJ (2012) Nitric oxide as a regulator of behavior: new ideas from Aplysia feeding. Prog Neurobiol 97(3):304–317. https://doi.org/10.1016/j.pneurobio.2012.03.004
Sutton GP, Macknin JB, Gartman SS, Sunny GP, Beer RD, Crago PE, Neustadter DM, Chiel HJ (2004) Passive hinge forces in the feeding apparatus of Aplysia aid retraction during biting but not during swallowing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 190(6):501–514. https://doi.org/10.1007/s00359-004-0517-4
Sutton GP, Mangan EV, Neustadter DM, Beer RD, Crago PE, Chiel HJ (2004) Neural control exploits changing mechanical advantage and context dependence to generate different feeding responses in Aplysia. Biol Cybern 91(5):333–345. https://doi.org/10.1007/s00422-004-0517-z
Szczecinski NS, Chrzanowski DM, Cofer DW, Terrasi AS, Moore DR, Martin JP, Ritzmann RE, Quinn RD (2015) Introducing mantisbot: Hexapod robot controlled by a high-fidelity, real-time neural simulation. In: IEEE international conference on intelligent robots and systems, 2015-December (September), pp 3875–3881. https://doi.org/10.1109/IROS.2015.7353922
Szczecinski NS, Hunt AJ, Quinn RD (2017) A functional subnetwork approach to designing synthetic nervous systems that control legged robot locomotion. Front Neurorobotics. https://doi.org/10.3389/fnbot.2017.00037
Szczecinski NS, Quinn RD (2018) Leg-local neural mechanisms for searching and learning enhance robotic locomotion. Biol Cybern 112(1–2):99–112. https://doi.org/10.1007/s00422-017-0726-x
Tal D, Schwartz EL (1997) Computing with the leaky integrate-and-fire neuron: logarithmic computation and multiplication. Neural Comput 9(2):305–318
Teyke T, Weiss KR, Kupfermann I (1991) Activity of identified cerebral neuron correlates with food-induced arousal in Aplysia. Neurosci Lett 133(2):307–310. https://doi.org/10.1016/0304-3940(91)90595-K
Verstappen M, Aerts P, Van Damme R (2000) Terrestrial locomotion in the black-billed magpie: kinematic analysis of walking, running and out-of-phase hopping. J Exp Biol 203(14):2159–2170
Wang Y, Truccolo W, Borton DA (2018) Decoding hindlimb kinematics from primate motor cortex using long short-term memory recurrent neural networks. In: Proceedings of the annual international conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2018-July, 1944–1947. https://doi.org/10.1109/EMBC.2018.8512609
Warman EN, Chiel HJ (1995) A new technique for chronic single-unit extracellular recording in freely behaving animals using pipette electrodes. J Neurosci Methods 57(2):161–169. https://doi.org/10.1016/0165-0270(94)00144-6
Webster VA, Lonsberry AJ, Horchler AD, Shaw KM, Chiel HJ, Quinn RD (2013) A segmental mobile robot with active tensegrity bending and noise-driven oscillators. In: 2013 IEEE/ASME international conference on advanced intelligent mechatronics: mechatronics for human wellbeing, AIM 2013. Wollongong, Australia, pp 1373–1380. https://doi.org/10.1109/AIM.2013.6584286
Weiss KR, Chiel HJ, Koch U, Kupfermann I (1986) Activity of an identified histaminergic neuron, and its possible role in arousal of feeding behavior in semi-intact Aplysia. J Neurosci 6(August):2403–2415
Wilson HR, Cowan JD (1972) Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J 12(1):1–24. https://doi.org/10.1016/S0006-3495(72)86068-5
Wilson HR, Cowan JD (1973) A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik 13(2):55–80. https://doi.org/10.1007/BF00288786
Wood KC, Blackwell JM, Geffen MN (2017) Cortical inhibitory interneurons control sensory processing. Curr Opin Neurobiol 46:200–207. https://doi.org/10.1016/j.conb.2017.08.018
Xie Z, Schwartz O, Prasad A (2018) Decoding of finger trajectory from ECoG using deep learning. J Neural Eng. https://doi.org/10.1088/1741-2552/aa9dbe
Ye H, Morton DW, Chiel HJ (2006) Neuromechanics of coordination during swallowing in Aplysia californica. J Neurosci 26(5):1470–1485. https://doi.org/10.1523/JNEUROSCI.3691-05.2006
Ye H, Morton DW, Chiel HJ (2006) Neuromechanics of multifunctionality during rejection in Aplysia californica. J Neurosci 26(42):10743–10755
Yu SN, Crago P, Chiel H (1997) A nonisometric kinetic model for smooth muscle. Am J Physiol Cell Physiol 272(3):C1025–C1039
Yu SN, Crago PE, Chiel HJ (1999) Biomechanical properties and a kinetic simulation model of the smooth muscle I2 in the buccal mass of Aplysia. Biol Cybern 81:505–513. https://doi.org/10.1007/s004220050579
Zahalak GI, Ma SP (1990) Muscle activation and contraction: constitutive relations based directly on cross-bridge kinetics. J Biomech Eng 112(1):52–62. https://doi.org/10.1115/1.2891126
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17(4):359–411
Ziv I, Baxter DA, Byrne JH (1994) Simulator for neural networks and action potentials: description and application. J Neurophysiol 71(1):294–308
Acknowledgements
HJC and JPG were supported by NSF Grant IOS1754869. VWW was supported by startup funding from the Carnegie Mellon University Department of Mechanical Engineering. VWW, HJC, and JPG were supported by NSF Grant DBI2015317. HJC and PJT were supported by NIH Grant R01 NS118606. PJT thanks Oberlin College for research support. We thank the anonymous reviewers for helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Benjamin Lindner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendices
1.1 Semi-implicit integration scheme
Suppose a continuously varying quantity x satisfies the initial value problem
where \(x_\infty (t)\) is set by the other variables in our system, say \(\{y_i\}_{i=2}^n\), generally following some nonlinear dependencies, and \(\tau \) is a fixed time constant. We would like to implement a numerical approximation to the exact solution for x, namely
along with the remaining variables that satisfy their own differential equations. Euler’s forward method is convenient to implement but prone to numerical instability. Euler’s backward or implicit method is numerically stable but computationally expensive, as it requires solving an implicit equation at each step. Both methods proceed from a discrete approximation of the derivative, namely
In both cases we create an update rule \(x(t)\rightarrow x(t+h)\), by evaluating the right-hand side of (13) at either time t or time \(t+h\), and solving for \(x(t+h)\).
Forward:
Backward:
Since the variables \(y_2,\ldots ,y_n\) appear on the right-hand side of (17) evaluated at the later time point, \(t+h\), (17) is part of a system of n nonlinear equations that must be solved simultaneously to determine the system state at \(t+h\). Both numerical schemes (15) and (17) are first-order accurate, meaning that the truncation error between the true solution (12) and the numerical approximation scales as \(\mathcal {O}(h^2)\) on each time step, with a global error (after T/h time steps for a simulation of total runtime T) that is \(\mathcal {O}(h)\).
Semi-implicit: In our model implementation, we use a semi-implicit method based on the approximation
namely

At each time step we update x using a weighted average of its past value x(t) and its target value \(x_\infty (t)\), with the (short) timestep h and the intrinsic time constant \(\tau \) providing the relative weight of past and future. We expect an accurate approximation to (12) provided \(h\ll \tau \). As we show below, the method is first-order accurate, and numerically stable, but it does not require solving an implicit equation at each time step. Thus, this method combines the advantages of both the forward and backward methods. The method may be seen as an example of operator splitting.[139]
To see that (19) is first-order accurate, we assume that x(t) is smooth enough to have Taylor expansions through the second order. Thus, for \(h\ll 1\) we may write
Thus, the semi-implicit scheme (19) is first-order accurate in the time step h.
To see that (19) is numerically stable, suppose that we fix \(\mathbf {y}\) so that \(x_\infty (\mathbf {y})=c,\) a constant. Clearly if \(x(t)=c\) then \(x(t+h)=c\) as well, so \(x=c\) is a fixed point of the iteration (19), under this assumption. Numerical stability follows if we can show that \(x=c\) is a stable fixed point for all \(h>0\), as we now establish. Let \(x(t_0+nh)=c+a_n,\) with \(a_0\) arbitrary. Then
no matter the size of the timestep \(h>0\). Thus, the scheme (19) is both (first-order) accurate and numerically stable.
The head and grasper position variables \(x_\text {h}\), \(x_\text {g}\) form a linearly coupled pair, for which we can extend the semi-implicit algorithm given in one-dimensional form above. In general, consider a nonhomogeneous linear system expressed in terms of a vector \(\mathbf {x}\), a matrix A, and a forcing vector \(\mathbf {b}\):
To set up a semi-implicit first-order iteration scheme, observe that
Dropping the \(\mathcal {O}(h^2)\) term and writing the update scheme in MATLAB style notation gives

(Here the backslash notation \(M\backslash \mathbf {u}\) stands for \(M^{-1}\mathbf {u}\), i.e., the least-squares solution \(\mathbf {y}\) to the linear system \(M\mathbf {y}=\mathbf {u}\).) Comparing this update scheme with (19), it is easy to check that they are consistent for a single variable by setting \(A=-1/\tau \) and \(b=x_\infty /\tau \).
In our case the head and grasper positions \(x_\text {h}\) and \(x_\text {g}\) comprise a linearly coupled system, \(\mathbf {x}\), and the coupling matrix A is \(2\times 2\), so \(I-hA\) can be inverted explicitly, provided h is smaller than the reciprocal of the largest positive eigenvalue of A. (If no eigenvalues of A are positive real numbers, then \(I-hA\) can always be inverted.) For a general \(2\times 2\) system the update rule reads
with all time-varying elements of the right-hand side evaluated at time t. In (35) \(\text {Tr}A\) and \(\det A\) denote the trace and determinant of A, respectively. Thus, truncating terms of order \(\mathcal {O}(h^2)\) and higher gives a first-order semi-implicit update scheme for two-component state and forcing vectors \(\mathbf {x}\) and \(\mathbf {b}\):

In (36) I denotes the \(2\times 2\) identity matrix.
1.2 Table of symbols
Table 3 provides a table of symbols.
1.3 Boolean logic of Aplysia feeding control
The following sections detail the logic implementations for the activity of each neuron in the controller network. All neurons, except for B4/B5, are implemented as standard Boolean elements and are either OFF (0), or ON (1). B4/B5 is a ternary unit which is either OFF (0), weakly ON (1), or strongly ON (2). The activity state of B4/B5 must be checked using conditional logic prior to negation, as \(\,!\,N_{B4/B5}\) is undefined (see Sect. 3.5). These interactions include known direct connections between neurons based on previous literature as well as hypothesized connections that may be direct or indirect, and indirect sensory feedback pathways. Sensory feedback pathways involving proprioception of the grasper position and pressure exerted by the closed grasper are gated by logic tests of position and pressure relative to user-specified thresholds. Allowing these thresholds to vary in response to activity levels of interneurons and external sensory cues provides an approximation of neuromodulation. All time varying elements on the right side of the logic equations are at time (j).
Cerebral Interneurons
-
1.
Metacerebral Cell
$$\begin{aligned} N_\text {MCC}(j+1) = [\text {arousal}] \end{aligned}$$(37) -
2.
CBI-2
CBI-2 is activated by sensory inputs present in biting and rejection, but not in swallowing.
$$\begin{aligned} \begin{aligned}&N_\text {CBI-2}(j+1) \\&\quad = N_\text {MCC}\,\,(\,!\,N_\text {B64}) \,\,\big (\left( [\text {lips}_\text {mech}]\,\,[\text {lips}_\text {chem}]\,\,\,!\,[\text {grasper}_\text {mech}]\right) \parallel \\&\qquad \left( [\text {grasper}_\text {mech}]\,\,\,!\,[\text {lips}_\text {chem}]\right) \big ) \end{aligned} \end{aligned}$$(38)With the hypothesized connections in Sect. 4.3, these equations change to:
$$\begin{aligned} \begin{aligned}&N_\text {CBI-2}(j+1) \\&\quad = N_\text {MCC}\,\,(\,!\,N_\text {B64}) \,\,\big (\left( [\text {lips}_\text {mech}]\,\,[\text {lips}_\text {chem}]\,\,\,!\,[\text {grasper}_\text {mech}]\right) \parallel \\&\qquad \left( [\text {grasper}_\text {mech}]\,\,\,!\,[\text {lips}_\text {chem}]\right) \parallel \left( N_\text {B4/B5}\ge 2 \right) \big ) \end{aligned} \end{aligned}$$(39) -
3.
CBI-3
CBI-3 is activated by sensory inputs present in biting and swallowing, but not in rejection.
$$\begin{aligned} \begin{aligned} N_\text {CBI-3}(j+1) =&N_\text {MCC}\,\,[\text {lips}_\text {mech}]\,\,[\text {lips}_\text {chem}]\end{aligned} \end{aligned}$$(40)With the equations and refractory period proposed in Sect. 4.3, the logic implementation for CBI-3 changes to include a gating state variable based on whether or not the neuron is in a refractory state following strong inhibition. Similar logic could be added to other nodes in the network as needed based on animal experiments. This period was included here as part of the hypothesis that strong activation of B4/B5 triggers rejection in animals that are swallowing. This hypothesis and an assessment of whether this refractory period occurs in CBI-3 in animal preparations or whether this effect is due to another mechanism could be tested experimentally. The equation becomes:
$$\begin{aligned} \begin{aligned} N_\text {CBI-3}(j+1) = \,&N_\text {MCC}\,\,[\text {lips}_\text {mech}]\,\,[\text {lips}_\text {chem}]\\&\,\,\left( N_\text {B4/B5}< 2 \right) \,\,\left( \,!\,[\text {refractory}_\text {CBI-3}]\right) \end{aligned} \end{aligned}$$(41) -
4.
CBI-4
CBI-4 is activated by sensory inputs present in swallowing and rejection, but not in biting.
$$\begin{aligned} N_\text {CBI-4}(j+1) = N_\text {MCC}\,\,\left( [\text {lips}_\text {mech}]\parallel [\text {lips}_\text {chem}]\right) \,\,[\text {grasper}_\text {mech}]\end{aligned}$$(42)
Buccal Interneurons
-
1.
B64
Activity in \(N_\text {B64}\) is influenced by the activity of the \(N_\text {MCC}\) and \(N_\text {B31/B32}\). It is also excited by protraction and inhibited by retraction. The proprioceptive feedback is implemented as:
$$\begin{aligned}&\text {B64}_\text {proprioception} \nonumber \\&\quad = (N_\text {CBI-3}\,\,(([\text {grasper}_\text {mech}]\,\,[\text {protracted}_{N_\text {B64},\text {swallow}}]) \parallel \nonumber \\&\qquad ((\,!\,[\text {grasper}_\text {mech}])\,\,[\text {protracted}_{N_\text {B64},\text {bite}}]))\quad ) \parallel \nonumber \\&\qquad ((\,!\,N_\text {CBI-3})\,\,[\text {protracted}_{N_\text {B64},\text {reject}}]) \end{aligned}$$(43)where
$$\begin{aligned}&\left[ \text {protracted}_{N_\text {B64},\text {swallow}}\right] = [x_{\text {g/h}}> z_{\text {B64},\text {swallow}}] \end{aligned}$$(44)$$\begin{aligned}&\left[ \text {protracted}_{N_\text {B64},\text {bite}}\right] = [x_{\text {g/h}}> z_{\text {B64},\text {bite}}] \end{aligned}$$(45)$$\begin{aligned}&\left[ \text {protracted}_{N_\text {B64},\text {reject}}\right] = [x_{\text {g/h}}> z_{\text {B64},\text {reject}}] \end{aligned}$$(46)This amounts to the threshold being depend on the behavior with different threshold values for bites, swallows, and rejections.
$$\begin{aligned} \begin{aligned} N_\text {B64}(j+1) =&N_\text {MCC}\,\,(\,!\,N_\text {B31/B32}) \,\,\text {B64}_\text {proprioception} \end{aligned} \end{aligned}$$(47) -
2.
B4/B5
\(N_\text {B4/B5}\) has been shown to have varying effects when firing strongly versus weakly. To represent this in the modeling framework, quiescence is represented as 0, weak firing as 1 and strong firing as 2. The neurons are quiescent during biting, and they fire weakly during the retraction phase of swallowing. The neurons fire strongly when stimulated with the external electrode and during the retraction phase of rejection. During rejection, B4/B5 is observed to cease firing, allowing B3/B6/B9 to fire briefly at the end of the behavior. To implement this, we have used a proprioceptive feedback pathway which inhibits the activity of B4/B5 once the grasper has reached a user-specified level of retraction.
$$\begin{aligned} N_\text {B4/B5}(j+1)&= N_\text {MCC}\,\,\bigg ( (\,!\,[\text {electrode}_\text {B4/B5}]) \,\,\big ( 2 (\,!\,N_\text {CBI-3}) \,\,N_\text {B64}\nonumber \\&\quad \,\,[\text {protracted}_{N_\text {B4/B5}}] \nonumber \\&\quad +N_\text {CBI-3}\,\,[\text {grasper}_\text {mech}]\,\,N_\text {B64}\big ) \nonumber \\&\quad +2 \, [\text {electrode}_\text {B4/B5}] \bigg ) \end{aligned}$$(48)where
$$\begin{aligned}{}[\text {protracted}_{N_\text {B4/B5}}] = [x_{\text {g/h}}> z_{\text {B4/B5}}] \end{aligned}$$(49) -
3.
B20
$$\begin{aligned} \begin{aligned}&N_\text {B20}(j+1) \\&\quad = N_\text {MCC}\big (N_\text {CBI-2}\parallel N_\text {CBI-4}\parallel N_\text {B31/B32}\big ) \,\,\,!\,N_\text {CBI-3}\,\,\,!\,N_\text {B64}\end{aligned} \end{aligned}$$(50) -
4.
B40/B30
\(N_\text {B40/B30}\) has fast inhibitory and slow excitatory connections to \(N_\text {B8}\). To capture this, we record the time (j) at which \(N_\text {B40/B30}\) transitions between states for later use in the \(N_\text {B8}\) activity calculations (see below). First, the activity of \(N_\text {B40/B30}\) in the next time step is determined:
$$\begin{aligned} \begin{aligned} N_\text {B40/B30}(j+1) = N_\text {MCC}\,\,\big (N_\text {CBI-2}\parallel N_\text {CBI-4}\parallel N_\text {B31/B32}\big ) \,\,\,!\,N_\text {B64}\end{aligned} \end{aligned}$$(51)After calculating the new activity, we assess transitions as defined by the following pseudocode:
if \((N_\text {B40/B30}(j) == 0 \, \text {AND} \, N_\text {B40/B30}(j+1) == 1)\), then set \(t_{N_\text {B40/B30}\text {,on}}\) = j;
if \((N_\text {B40/B30}(j) == 1 \, \text {AND} \, N_\text {B40/B30}(j+1) == 0)\), then set \(t_{N_\text {B40/B30}\text {,off}}\) = j;
Buccal Motor Neurons
-
1.
B31/B32
\(N_\text {B31/B32}\) receives input from interneurons and proprioceptive feedback. To capture possible modulation of \(N_\text {B31/B32}\) and generate multifunctional behavior under different sensory cues, behavior-dependent proprioceptive inputs are implemented. Though the resulting full equation for \(N_\text {B31/B32}\) activity is large, it can be broken down to three sections: (1) if \(N_\text {CBI-3}\) is active and there is sensory stimuli in the grasper (swallowing), (2) if \(N_\text {CBI-3}\) is active and there is NOT sensory stimuli in the grasper (biting), and (3) if \(N_\text {CBI-3}\) is NOT active (rejection).
$$\begin{aligned}&N_\text {B31/B32}(j+1) \nonumber \\&\quad = N_\text {MCC}\,\,\bigg ( N_\text {CBI-3}\,\,\nonumber \\&[\text {grasper}_\text {mech}]( (\,!\,N_\text {B64}) \,\,((![\text {pressure}_{N_\text {B31/B32},\text {ingestion}}]) \parallel N_\text {CBI-2}) \,\,\nonumber \\&\quad ((\,!\,N_\text {B31/B32})\,\,[\text {retracted}_{N_\text {B31/B32},\text {swallow},\text {off}}] +\nonumber \\&\quad N_\text {B31/B32}\,\,[\text {retracted}_{N_\text {B31/B32},\text {swallow},\text {on}}]))\nonumber \\&(\,!\,[\text {grasper}_\text {mech}]) ( (\,!\,N_\text {B64}) \,\,((![\text {pressure}_{N_\text {B31/B32},\text {ingestion}}]) \parallel N_\text {CBI-2}) \,\,\nonumber \\&\quad ((\,!\,N_\text {B31/B32})\,\,[\text {retracted}_{N_\text {B31/B32},\text {bite},\text {off}}] +\nonumber \\&\quad N_\text {B31/B32}\,\,[\text {retracted}_{N_\text {B31/B32},\text {bite},\text {on}}])) + \nonumber \\&(\,!\,N_\text {CBI-3}) \,\,\big ((\,!\,N_\text {B64}) \,\,[\text {pressure}_{N_\text {B31/B32},\text {rejection}}] \,\,\nonumber \\&\quad (N_\text {CBI-2}\parallel N_\text {CBI-4}) \,\,\nonumber \\&\quad ((\,!\,N_\text {B31/B32})\,\,[\text {retracted}_{N_\text {B31/B32},\text {reject},\text {off}}] + \nonumber \\&\quad N_\text {B31/B32}\,\,[\text {retracted}_{N_\text {B31/B32},\text {reject},\text {on}}] \big ) \bigg ) \end{aligned}$$(52)where
$$\begin{aligned}&{[}\text {pressure}_{N_\text {B31/B32},\text {ingestion}}] = [P_g > 0.5 p_\text {max}] \end{aligned}$$(53)$$\begin{aligned}&{[}\text {pressure}_{N_\text {B31/B32},\text {rejection}}] = [P_g > 0.25 p_\text {max}] \end{aligned}$$(54)$$\begin{aligned}&{[}\text {retracted}_{N_\text {B31/B32},\text {swallow},\text {off}}] = [x_{\text {g/h}}< z_{N_\text {B31/B32},\text {swallow},\text {off}}]\nonumber \\ \end{aligned}$$(55)$$\begin{aligned}&{[}\text {retracted}_{N_\text {B31/B32},\text {swallow},\text {on}}] = [x_{\text {g/h}}< z_{N_\text {B31/B32},\text {swallow},\text {on}}] \nonumber \\ \end{aligned}$$(56)$$\begin{aligned}&{[}\text {retracted}_{N_\text {B31/B32},\text {bite},\text {off}}] = [x_{\text {g/h}}< z_{N_\text {B31/B32},\text {bite},\text {off}}] \nonumber \\ \end{aligned}$$(57)$$\begin{aligned}&{[}\text {retracted}_{N_\text {B31/B32},\text {bite},\text {on}}] = [x_{\text {g/h}}< z_{N_\text {B31/B32},\text {bite},\text {on}}]\nonumber \\ \end{aligned}$$(58)$$\begin{aligned}&{[}\text {retracted}_{N_\text {B31/B32},\text {reject},\text {off}}] = [x_{\text {g/h}}< z_{N_\text {B31/B32},\text {reject},\text {off}}]\nonumber \\ \end{aligned}$$(59)$$\begin{aligned}&{[}\text {retracted}_{N_\text {B31/B32},\text {reject},\text {on}}] = [x_{\text {g/h}}< z_{N_\text {B31/B32},\text {reject},\text {on}}] \end{aligned}$$(60) -
2.
B6/B9/B3
$$\begin{aligned} \begin{aligned}&N_\text {B6/B9/B3}(j+1) \\&\quad = N_\text {MCC}\,\,N_\text {B64}\,\,(\,!\,(N_\text {B4/B5}\ge 2)) \,\,\\&\bigg (\big ((N_\text {CBI-3}\,\,(\,!\,[\text {grasper}_\text {mech}])) \,\,[\text {pressure}_{N_\text {B6/B9/B3},\text {bite}}] \big ) + \\&\big ((N_\text {CBI-3}\,\,[\text {grasper}_\text {mech}]) \,\,[\text {pressure}_{N_\text {B6/B9/B3},\text {swallow}}] \big ) + \\&(\,!\,N_\text {CBI-3}) \,\,(\,!\,[\text {pressure}_{N_\text {B6/B9/B3},\text {reject}}]) \big ) \bigg ) \end{aligned} \end{aligned}$$(61)where
$$\begin{aligned}&{[}\text {pressure}_{N_\text {B6/B9/B3},\text {bite}}] \nonumber \\&\quad = [P_g > z_{N_\text {B6/B9/B3},\text {bite},\text {pressure}})] \end{aligned}$$(62)$$\begin{aligned}&[\text {pressure}_{N_\text {B6/B9/B3},\text {swallow}}] \nonumber \\&\quad = [P_g > z_{N_\text {B6/B9/B3},\text {swallow},\text {pressure}})] \end{aligned}$$(63)$$\begin{aligned}&{[}\text {pressure}_{N_\text {B6/B9/B3},\text {reject}}] \nonumber \\&\quad = [P_g > z_{N_\text {B6/B9/B3},\text {reject},\text {pressure}}] \end{aligned}$$(64) -
3.
B8a/b
\(N_\text {B8}\) receives fast inhibitory and slow excitatory input from \(N_\text {B40/B30}\) [33, 79]. In the Boolean framework here we implement this as an excitatory input immediately following cessation of \(N_\text {B40/B30}\) activity for a user-specified duration (\(\text {duration}_{N_\text {B40/B30},\text {excite}}\)). Prior to calculating a new value for \(N_\text {B8}\), we first check whether the synaptic connection from \(N_\text {B40/B30}\) is excitatory with the following statements:
if \((N_\text {B40/B30}(j) == 0 \, \text {AND} \, j < (t_{N_\text {B40/B30}\text {,off}} + \text {duration}_{N_\text {B40/B30},\text {excite}}))\), then set \(N_\text {B40/B30},\text {excite} = 1\)
else set \(N_\text {B40/B30},\text {excite} = 0\)
$$\begin{aligned} \begin{aligned} N_\text {B8}(j+1) =&N_\text {MCC}\,\,(\,!\,(N_\text {B4/B5}\ge 2)) \,\,\\&((N_\text {CBI-3}\,\,(N_\text {B20}\parallel (N_\text {B40/B30},\text {excite})) \,\,\\&\quad \quad (\,!\,N_\text {B31/B32})) + \\&\quad ((\,!\,N_\text {CBI-3}) \,\,N_\text {B20})) \end{aligned} \end{aligned}$$(65) -
4.
B7
$$\begin{aligned} \begin{aligned}&N_\text {B7}(j+1) = N_\text {MCC}\\&\quad \,\,\big (\big ((\,!\,N_\text {CBI-3}\parallel [\text {grasper}_\text {mech}]) \\&\quad \,\,([\text {protracted}_{N_\text {B7},\text {reject}}] \parallel [\text {pressure}_{N_\text {B7}}])\big ) + \\&\big ((N_\text {CBI-3}\,\,\,!\,[\text {grasper}_\text {mech}]) \,\,([\text {protracted}_{N_\text {B7},\text {bite}}] \\&\quad \parallel [\text {pressure}_{N_\text {B7}}])\big )\big ) \end{aligned} \end{aligned}$$(66)where
 (67)
(67) (68)
(68)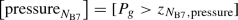 (69)
(69) -
5.
B38
$$\begin{aligned} \begin{aligned} N_\text {B38}(j+1) = N_\text {MCC}\,\,[\text {grasper}_\text {mech}]\,\,\bigg ( N_\text {CBI-3}\,\,[\text {retracted}_{N_\text {B38}}] \bigg ) \end{aligned} \end{aligned}$$(70)where
$$\begin{aligned} {[}\text {retracted}_{N_\text {B38}}] = [x_{\text {g/h}}< z_{\text {B38}}] \end{aligned}$$(71)
1.4 Muscle forces
Contact forces, such as the pressure resulting from grasper closure and force due to the anterior pinch, are implemented as second-order responses to neural activation using the semi-implicit integration scheme, Eq. (19), as shown in the following equations.
-
1.
Grasper Pressure
$$\begin{aligned}&P_\text {I4}(t+h) = \frac{\tau _\text {I4} P_\text {I4}(t) + h A_\text {I4}(t)}{\tau _\text {I4} + h} \end{aligned}$$(72)$$\begin{aligned}&A_\text {I4}(t+h) = \frac{\tau _\text {I4} A_\text {I4}(t) + h N_\text {B8}(t)}{\tau _\text {I4} + h} \end{aligned}$$(73) -
2.
Pinch Pressure
$$\begin{aligned}&P_\text {I3,ant.}(t+h) = \frac{\tau _\text {I3,ant.} P_\text {I3,ant.} (t) + h A_\text {I3,ant.}(t) }{\tau _\text {I3,ant.} +h} \end{aligned}$$(74)$$\begin{aligned}&A_\text {I3,ant.}(t+h) \nonumber \\&\quad = \frac{\tau _\text {I3,ant.} A_\text {I3,ant.}(t) + h (N_\text {B38}(t) + N_\text {B6/B9/B3}(t))}{\tau _\text {I3,ant.} + h}\nonumber \\ \end{aligned}$$(75)
Muscle tensions for the remaining musculature were calculated using a second-order response to the neural activity as outlined in the following equations.
-
1.
I3 Tension
$$\begin{aligned}&T_\text {I3}(t+h) = \frac{\tau _\text {I3} T_\text {I3}(t) + h A_\text {I3}(t)}{\tau _\text {I3} + h} \end{aligned}$$(76)$$\begin{aligned}&A_\text {I3}(t+h) = \frac{\tau _\text {I3} A_\text {I3}(t) + h N_\text {B6/B9/B3}(t)}{\tau _\text {I3} + h} \end{aligned}$$(77) -
2.
I2 Tension
Time constants for I2 were tuned independently for ingestion and egestion to account for the experimental observations that egestions have a longer period than ingestions. Such variation in responsiveness of the animal may exist due to differences in neuromodulation between the behaviors. Therefore, the time constant for I2 is calculated as:
$$\begin{aligned}&\tau _\text {I2} =N_\text {CBI-3}\;\tau _\text {I2,ingestion} \nonumber \\&\qquad + (\,!\,N_\text {CBI-3}) \;\tau _\text {I2,egestion} \end{aligned}$$(78)$$\begin{aligned}&T_\text {I2}(t+h) = \frac{\tau _\text {I2} T_\text {I2} + h A_\text {I2}}{\tau _\text {I2} + h} \end{aligned}$$(79)$$\begin{aligned}&A_\text {I2}(t+h) = \frac{\tau _\text {I2} A_\text {I2}(t) + h N_\text {B31/B32}(t)}{\tau _\text {I2} + h} \end{aligned}$$(80) -
3.
Hinge Tension
$$\begin{aligned} T_\text {hinge}(t+h)= & {} \frac{\tau _\text {hinge} T_\text {hinge}(t) + h A_\text {hinge}(t)}{\tau _\text {hinge} + h} \end{aligned}$$(81)$$\begin{aligned} A_\text {hinge}(t+h)= & {} \frac{\tau _\text {hinge} A_\text {hinge}(t) + h N_\text {B7}(t)}{\tau _\text {hinge} + h} \end{aligned}$$(82)
1.5 Biomechanical model
The motions of the head and grasper are calculated based on the quasi-static equations of motion:
where \(x_\text {h}\) is the position of the head relative to the ground frame, \(x_\text {g}\) is the position of the grasper relative to the ground frame, and \(c_h\) and \(c_g\) are the damping coefficients for the motion of the head and grasper, respectively. The forces on the grasper and head can be calculated as outlined in the following sections.
1.5.1 Forces on the grasper
The positive direction for \(x_\text {g}\) corresponds to protraction (Fig. 3). The sum of forces on the grasper is
where the component forces are defined and calculated as follows:
\({F}_\text {I2}\): The force due to the I2 muscle. This value is dependent on the tension of the muscle as well as the mechanical advantage. It is scaled by a tunable maximum parameter, \(F_\text {I2,max}\), and is calculated as follows:
where \(x_{\text {g/h}}= x_\text {g}- x_\text {h}\) is the position of the grasper relative to the head.
\(F_{sp,g}\): The force in the spring connecting the grasper to the head. This spring represents the surrounding musculature of the esophagus, buccal mass, and extrinsic muscles which are not explicitly modeled. This is calculated as:
where \(K_g\) is the spring constant and \(x_{g/h}^0\) is the rest length of the spring.
\({F}_\text {I3}\): The force due to the I3 muscle which pushes the grasper backwards during retraction. This force is due to tension in I3 closing the muscular toroids. This value is dependent on the tension of the muscle as well as the mechanical advantage. It is scaled by a tunable maximum parameter, \(F_\text {I3,max}\), and is calculated as follows:
\({F}_\text {hinge}\): The force due to the hinge. This value is dependent on the tension of the muscle as well as the mechanical advantage. It is scaled by a tunable maximum parameter, \(F_\text {hinge,max}\), and is calculated as follows:
where \([\text {hinge stretched}] = [x_{\text {g/h}}>0.5]\) determines whether the hinge is sufficiently stretched to produce any force [148].
\(F_{f,g}\): Friction resulting from the grasper closing on an object. To determine \(F_{f,g}\) it is necessary to check if the grasper is slipping against the object by checking the inequality:
where \(\mu _{s,g}\) is the coefficient for static friction between the grasper and the object. \({F}_\text {I4}\) is the normal force due to the grasper muscle I4 closing on the object. This is calculated directly as the grasper pressure defined in the previous appendix applied to a unit area scaled by a parameter.
If the condition in Eq. (89) is true, then the contact is in a state of static friction and \(F_{f,g}\) is calculated as:
If the condition in Eq. (89) is not true, the contact is sliding and is in a state of kinetic friction, and \(F_{f,g}\) is calculated as:
where \(\mu _{k,g}\) is the coefficient for kinetic friction between the grasper and the seaweed.
The sign of the friction force is dependent on which direction the grasper would be moving without the friction present, and \({F}_{f,g}\) can be calculated as:
1.5.2 Forces on head
The forces on the head are calculated as:
The muscles and grasper spring exert forces on the head equal and opposite to those on the grasper. As the muscles contract and apply forces to move the grasper forward, this also stretches the spring between the grasper and head proportionally to the muscle force. For the quasi-static model, acceleration is assumed to be negligible and therefore the forces on the grasper must equal zero.
Solving for the spring forces, \(F_{sp,g}\), and substituting into Eq. (94) yields:

which simplifies to:
where \(F_{sp,h}\) is the spring force between the head and neck of the animal, \(F_{f,g}\) is the previously calculated friction force between the grasper and the object, and \(F_{f,h}\) is the friction force resulting from the jaws pinching on the object. These components are calculated as follows.
where \(K_h\) is the spring constant and \(x_{h}^0\) is the rest length of the spring.
To determine the value of \(F_{f,h}\) it is necessary to check if the jaws are slipping relative to the seaweed by checking the following inequality:
where \(\mu _{s,h}\) is the coefficient for static friction between the jaws and the seaweed. \({F}_\text {I3,ant.}\) is the normal force due to the anterior portion of the I3 jaw muscle closing on the seaweed. This is calculated directly as the pinch pressure defined in the previous appendix applied to a unit area, scaled by a parameter, and multiplied by a mechanical advantage term:
If the condition in Eq. (99) is true, the jaws are in static friction and \(F_{f,h}\) is calculated as:
If the condition in Eq. (99) is not true, the jaws are slipping and \(F_{f,h}\) is calculated as:
where \(\mu _{k,h}\) is the coefficient for kinetic friction between the jaws and the seaweed.
The sign of the friction force is dependent on which direction the head would be moving without the friction present and \({F}_{f,h}\) can be calculated as:
1.5.3 Force on objects
The force on the object if unbroken is equal to the sum of the friction forces where we use the conventions that positive force indicates tension on the force transducer:
If \(F_o \le z_s\), where \(z_s\) is the user defined seaweed strength, the seaweed is not broken and the motion of the bodies is calculated based on the forces calculated in the previous sections. If \(F_o > z_s\), the seaweed is broken and can no longer transmit forces to the head or grasper. Therefore, the forces on the head and grasper are recalculated as:
A Boolean tracking variable [unbroken] is used to track whether the seaweed is intact (1) or broken (0). Once the seaweed breaks, it is not restored until the grasper has completed a new protraction and grasp motion. For this model, we have implemented this by resetting \([\text {unbroken}] = 1\) if at the current timestep \([\text {unbroken}] ==0\) AND \(x_{\text {g/h}}< 0.3\) AND \(x_{\text {g/h}}(j+1)>x_{\text {g/h}}(j)\). These thresholds were tuned manually for this implementation.
1.5.4 Updating grasper and head positions
All of the forces in this biomechanical model are linearly dependent on the position of the head, \(x_\text {h}\), and grasper, \(x_\text {g}\). Therefore, they can each be rewritten in the form:
As a consequence, the equations of motion can be rewritten in the form
This can then be integrated with the semi-implicit integration scheme in Appendix A.1 as:
Rights and permissions
About this article
Cite this article
Webster-Wood, V.A., Gill, J.P., Thomas, P.J. et al. Control for multifunctionality: bioinspired control based on feeding in Aplysia californica. Biol Cybern 114, 557–588 (2020). https://doi.org/10.1007/s00422-020-00851-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-020-00851-9