Abstract
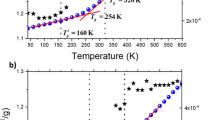
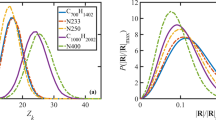
Molecular dynamics (MD) simulations are conducted to systematically benchmark the effects of molecular weight, chain number, and cooling rate on the glass transition temperature (Tg) and coefficient of thermal expansion (CTE) of poly(ethylene oxide) (PEO). Hyperbolic regression as an objective identified method is used to extract Tg and CTE. The results show that for a cooling rate higher than 5 × 1013 K/min, Tg and CTE are both strongly affected by rapid quenching. For a cooling rate lower than 5 × 1013 K/min, Tg and CTE in the high-temperature domain still slightly depend on the cooling rate. Eventually, to eliminate the finite size effect of the model, a threshold molecular weight of 11,240 g/mol should be satisfied in the system. In addition, the chain number must be more than 10, at least for an oligomer system (50 monomers).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ward IM, Hadley DW. An introduction to the mechanical properties of solid polymers. John Wiley & Sons, Chichester, UK; 1993.
Wu C. Simulated glass transition of poly (ethylene oxide) bulk and film: a comparative study. J Phys Chem B. 2011;115:11044–52.
Lukasheva N, Tolmachev D, Nazarychev V, Kenny J, Lyulin S. Influence of specific intermolecular interactions on the thermal and dielectric properties of bulk polymers: atomistic molecular dynamics simulations of Nylon 6. Soft matter. 2017;13:474–85.
Yang S, Qu J. Computing thermomechanical properties of crosslinked epoxy by molecular dynamic simulations. Polymer. 2012;53:4806–17.
Li M, Liu X, Qin J, Gu Y. Molecular dynamics simulation on glass transition temperature of isomeric polyimide. Express Polym Lett. 2009;3:665–75.
Li C, Medvedev GA, Lee E-W, Kim J, Caruthers JM, Strachan A. Molecular dynamics simulations and experimental studies of the thermomechanical response of an epoxy thermoset polymer. Polymer. 2012;53:4222–30.
Soldera A, Metatla N. Glass transition of polymers: atomistic simulation versus experiments. Phys Rev E. 2006;74:061803.
Barrat J-L, Baschnagel J, Lyulin A. Molecular dynamics simulations of glassy polymers. Soft Matter. 2010;6:3430–46.
Buchholz J, Paul W, Varnik F, Binder K. Cooling rate dependence of the glass transition temperature of polymer melts: molecular dynamics study. J Chem Phys. 2002;117:7364–72.
Fox TG Jr, Flory PJ. Second‐order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys. 1950;21:581–91.
Durand M, Meyer H, Benzerara O, Baschnagel J, Vitrac O. Molecular dynamics simulations of the chain dynamics in monodisperse oligomer melts and of the oligomer tracer diffusion in an entangled polymer matrix. J Chem Phys. 2010;132:194902.
Kausik R, Mattea C, Fatkullin N, Kimmich R. Confinement effect of chain dynamics in micrometer thick layers of a polymer melt below the critical molecular weight. J Chem Phys. 2006;124:114903.
Liang T, Yang X, Zhang X. Prediction of polyimide materials with high glass‐transition temperatures. Polym Phys. 2001;39:2243–51.
Pan R, Liu X, Zhang A, Gu Y. Molecular simulation on structure–property relationship of polyimides with methylene spacing groups in biphenyl side chain. Comput Mater Sci. 2007;39:887–95.
Lyulin SV, Larin SV, Gurtovenko AA, Nazarychev VM, Falkovich SG, Yudin VE, et al. Thermal properties of bulk polyimides: insights from computer modeling versus experiment. Soft Matter. 2014;10:1224–32.
Li C, Coons E, Strachan A. Material property prediction of thermoset polymers by molecular dynamics simulations. Acta Mechanica. 2014;225:1187–96.
Lyulin S, Gurtovenko A, Larin S, Nazarychev V, Lyulin A. Microsecond atomic-scale molecular dynamics simulations of polyimides. Macromolecules. 2013;46:6357–63.
Patrone PN, Dienstfrey A, Browning AR, Tucker S, Christensen S. Uncertainty quantification in molecular dynamics studies of the glass transition temperature. Polymer. 2016;87:246–59.
Haag R, Kratz F. Polymer therapeutics: concepts and applications. Angew Chem Int Ed. 2006;45:1198–215.
Blumberg AA, Pollack SS, Hoeve C. A poly (ethylene oxide)–mercuric chloride complex. J Polym Sci Part A Gen Pap. 1964;2:2499–502.
Tadokoro H. Structure of crystalline polyethers. J Polym Sci Macromol Rev. 1967;1:119–72.
Jiang Y, Yan X, Ma Z, Mei P, Xiao W, You Q, et al. Development of the PEO based solid polymer electrolytes for all-solid state lithium ion batteries. Polymers. 2018;10:1237.
Harris JM, editor. Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications. New York: Plenum Press; 1992.
Accelrys. Materials Studio. http://accelrys.com/products/collaborative-science/biovia-materials-studio/ 2016.
Plimpton S. Fast parallel algorithms for short-range molecular dynamics. J Comput Phys. 1995;117:1–19.
Nosé S. A unified formulation of the constant temperature molecular dynamics methods. J Chem Phys. 1984;81:511–9.
Hoover WG. Canonical dynamics: equilibrium phase-space distributions. Phys Rev A. 1985;31:1695.
Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys. 1981;52:7182.
Swope WC, Andersen HC, Berens PH, Wilson KR. A computer simulation method for the calculation of equilibrium constants for the formation of physical clusters of molecules: application to small water clusters. J Chem Phys. 1982;76:637–49.
Sun H, Mumby SJ, Maple JR, Hagler AT. An ab initio CFF93 all-atom force field for polycarbonates. J Am Chem Soc. 1994;116:2978–87.
Maple JR, Dinur U, Hagler AT. Derivation of force fields for molecular mechanics and dynamics from ab initio energy surfaces. Proc Natl Acad Sci. 1988;85:5350–4.
Stukowski A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model Simul Mater Sci Eng. 2009;18:015012.
Niedzwiedz K, Wischnewski A, Pyckhout-Hintzen W, Allgaier J, Richter D, Faraone A. Chain dynamics and viscoelastic properties of poly (ethylene oxide). Macromolecules. 2008;41:4866–72.
Yang H, Li Z-S, Qian H-J, Yang Y-B, Zhang X-B, Sun C-C. Molecular dynamics simulation studies of binary blend miscibility of poly(3-hydroxybutyrate) and poly(ethylene oxide). Polymer. 2004;45:453–7.
Luo Z, Jian J. Molecular dynamics and dissipative particle dynamics simulations for the miscibility of poly(ethylene oxide)/poly(vinyl chloride) blends. Polymer. 2010;51:291–9.
Brandrup J, Immerput EH, editors. Polymer handbook, 3rd ed. New York: Wiley; 1989.
Tsay SF, Liu CF. System-size effects in the molecular dynamics simulation of metallic crystallization. Physics Letters A. 1994;192:374–8.
Deng L, Du JC. Effects of system size and cooling rate on the structure and properties of sodium borosilicate glasses from molecular dynamics simulations. J. Chem. Phys. 2018;148:024504.
Mark JE. Polymer data handbook, New York: Oxford University Press; 2009.
Faucher J, Koleske J, Santee E Jr, Stratta J, Wilson C III. Glass transitions of ethylene oxide polymers. J Appl Phys. 1966;37:3962–4.
Vogel M. Conformational and structural relaxations of poly (ethylene oxide) and poly (propylene oxide) melts: molecular dynamics study of spatial heterogeneity, cooperativity, and correlated forward–backward motion. Macromolecules. 2008;41:2949–58.
Schmidtke B, Hofmann M, Lichtinger A, Rössler E. Temperature dependence of the segmental relaxation time of polymers revisited. Macromolecules. 2015;48:3005–13.
Bormuth A, Henritzi P, Vogel M. Chain-length dependence of the segmental relaxation in polymer melts: molecular dynamics simulation studies on poly (propylene oxide). Macromolecules. 2010;43:8985–92.
John V. Chang, editor. Frontiers in Condensed Matter Physics Research. New York: Nova Science Publishers, Inc.; 2006.
Doolittle AK. Studies in Newtonian flow. II. The dependence of the viscosity of liquids on free‐space. J Appl Phys. 1951;22:1471–5.
Cohen MH, Turnbull D. Molecular transport in liquids and glasses. J Chem Phys. 1959;31:1164–9.
Simha R, Boyer R. On a general relation involving the glass temperature and coefficients of expansion of polymers. J Chem Phys. 1962;37:1003–7.
Luo Z, Jian J. Molecular dynamics and dissipative particle dynamics simulations for the miscibility of poly(ethylene oxide)/poly(vinyl chloride) blends. Polymer. 2010;51:291–9.
Huang D, Simon SL, McKenna GB. Chain length dependence of the thermodynamic properties of linear and cyclic alkanes and polymers. J Chem Phys. 2005;122:084907.
Ribeiro CP Jr, Freeman BD. Sorption, dilation, and partial molar volumes of carbon dioxide and ethane in cross-linked poly (ethylene oxide). Macromolecules. 2008;41:9458–68.
Bicerano J. Prediction of polymer properties. New York: Marcel Dekker, Inc.; 2002.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology of Taiwan for financially supporting this research under Contract Nos. MOST 106-2622-E-492 -022 -CC3– and 107-2221-E-492 -011 -MY3. Support for significant computing resources from the NCHC in Taiwan is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, YC., Zhang, JF., Chiu, MH. et al. Molecular-weight and cooling-rate dependence of polymer thermodynamics in molecular dynamics simulation. Polym J 53, 455–462 (2021). https://doi.org/10.1038/s41428-020-00443-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00443-1