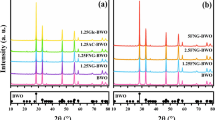
Abstract
TiO2 is a photocatalyst widely used for the degradation of organic compounds in aqueous media; however, it presents some difficulties for application on larger scales. The biggest limitations are its bandgap value of 3.2 eV which corresponds to the UV range of the spectrum and its difficult removal from the reaction medium after the reaction. An alternative to enhance the photocatalytic activity of TiO2 consists in design heterojunctions with semiconductors that are active under visible light irradiation. Some reports have described the magnetic property and synthesis procedures based on inexpensive and abundant raw material. In this work, we synthesized photocatalysts based on γ-Fe2O3impregnated with different levels of TiO2. This may contribute to improving the wide application of TiO2 in water, since γ-Fe2O3 has magnetic features that facilitate the removal of the catalyst after the reaction run. The materials were characterized by X-ray diffraction, analysis of adsorption/desorption of N2, reflectance diffuse, and 57Fe Mössbauer spectroscopy. The photocatalytic activity of the materials was tested for removing rhodamine under visible or visible light in the presence of H2O2. From the X-ray diffraction and 57Fe Mössbauer spectroscopy data, it was observed the formation of γ-Fe2O3 phase with small particle. Without visible light, only 45% of RhB was adsorbed, and with the light on, there is no increase in removal after 60 min. However, after adding H2O2, the photocatalytic activity of material was significantly improved, reaching 80% of dye removal. Tests using scavengers of reactive species revealed that −O2 and ․OH are the main species in this system. Moreover, the H2O2 retards the electron-hole recombination, thus increasing its photocatalytic activity. The ESI-MS analysis revealed that in 15 min of reaction, deethylation (m/z = 415, 388), deamination (m/z = 301 and 279), and rhodamine B severe oxidation products (m/z < 250) were present in solution, and TOC analysis confirms the mineralization of the rhodamine.









Similar content being viewed by others
References
Aldegs, Y., Barghouthi, M. I., El-sheikh, A. M., & Walker, G. M. (2008). Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes and Pigments, 77, 16–23.
Alhamedi, F. H., Rauf, M. A., & Ashraf, S. S. (2009). Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination, 238, 159–166.
Anbia, M., & Salehi, S. (2012). Removal of acid dyes from aqueous media by adsorption onto amino-functionalized nanoporous silica SBA-3. Dyes and Pigments, 94, 1–9.
Bharathi, S., Nataraj, D., Mangalaraj, D., Masuda, Y., Senthil, K., Yong, K. (2010). Highly mesoporous α-Fe2O3 nanostructures: preparation, characterization and improved photocatalytic performance towards Rhodamine B (RhB). Journal of Physics D: Applied Physics, 43(1), 1–17.
Crini, G. (2008). Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes and Pigments, 77(2), 415–426.
Cuiping, B., Xianfeng, X., Wenqi, G., Dexin, F., Mo, X., Zhongxue, G., & Nian, X. (2011). Removal of Rhodamine B by ozone-based advanced oxidation process. Desalination, 278, 84–90.
Ferreira, I. V. L., & Daniel, L. (2002). Fotocatálise heterogênea com TiO2 aplicada ao tratamento de esgoto sanitário secundário. Engenharia Sanitaria e Ambiental, 9(4), 335–342.
Forgacs, E., Cserháti, T., & Oros, G. (2004). Removal of synthetic dyes from wastewaters: a review. Environment International, 30(7), 953–971.
Gomathi Devi, L., & Kavitha, R (2013). A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Applied Catalysis B: Environmental, 140-141, 559–587.
Guo, S., Zhang, G., & Wang, J. (2014). Journal of Colloid and Interface Science, 433, 1–8.
Gupta, V. K., & Suhas. (2009). Application of low-cost adsorbents for dye removal--a review. Journal of Environmental Management, 90(8), 2313–2342.
Han, F., Kambala, V. S. R., Srinivasan, M., Rajarathnam, D., Naidu, D.(2009). Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Applied Catalysis A: General, 359(1-2), 25–40.
He, Z., Yang, S., Ju, Y., & Sun, C. (2009). Microwave photocatalytic degradation of Rhodamine B using TiO2 supported on activated carbon: mechanism implication. Journal of Environmental Sciences, 21(2), 268–272.
Hu, L., Yang, F., Lu, W., Hao, Y., & Yuan, H. (2013). Heterogeneous activation of oxone with CoMg/SBA-15 for the degradation of dye Rhodamine B in aqueous solution. Applied Catalysis B: Environmental, 134-135, 7–18.
Khenifi, A., Bouberka, Z., Sekrane, F., Kameche, M., & Derriche, Z. (2007). Adsorption study of an industrial dye by an organic clay. Adsorption, 13(2), 49–158.
Koch, C. B., Oxborrow, C. A., Morup, S., Madsen, M. B., Quinn, A. J., & Coey, J. M. D. (1995). Magnetic properties of feroxyhyte (δ-FeOOH). Physics and Chemistry of Minerals, 22, 333–341.
Konstantinou, I. K., & Albanis, T. A. (2004). TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review. Applied Catalysis B: Environmental, 49(1), 1–14.
Lee, D.-S., & Chen, Y.-W. (2014). Nano Ag/TiO2 catalyst prepared by chemical deposition and its photocatalytic activity. Journal of the Taiwan Institute of Chemical Engineers, 45(2), 705–712.
Lima, L. V., Rodriguez, M., Freitas, V. A., Souza, T. E., Machado, A. E., Patrocínio, A. O., Fabris, J. D., Oliveira, L. C. A., & Pereira, M. C. (2015). Synergism between n-type WO3 and p-type δ-FeOOH semiconductors: High interfacial contacts and enhanced photocatalysis. Applied Catalysis B: Environmental, 165, 579–588.
Lin, Y., Jiang, Z., Zhang, R., Zhu, C., Hu, X., Zhang, X., & Zhu, H. (2014). The structure, electronic, and optical properties of (Sm,N)-codoped anatase TiO2 photocatalyst: A density functional study. Journal of Catalysis, 309, 115–120.
Natarajan, T. S., Thomas, M., Natarajan, K., Bajaj, H. C., & Tayade, R. J. (2011). Study on UV-LED/TiO2 process for degradation of rhodamine B dye. Chemical Engineering Journal, 169(1-3), 126–134.
Online document Teixeira, C. P. A. B., & Jardim, W. D. F. (2004). Processos Oxidativos Avançados: Conceitos teóricos. Federal University of Campinas. http://lqa.iqm.unicamp.br/cadernos/caderno3.pdf. Acessed 16 Nov 2020.
Ortega-liebana, M. C., Hueso, J. L., Larrea, A., Sebastian, V., & Santamaria, J. (2015). Feroxyhyte nanoflakes coupled to up-converting carbon nanodots: a highly active, magnetically recoverable, Fenton-like photocatalyst in the visible-NIR range†. Chemical Communications, 51, 16625–16628.
Palanisamy, B., Babu, C., Sundaravel, B., Anandan, S., & Murugesan, V. (2013). Sol-gel synthesis of mesoporouus mixed Fe2O3/TiO2 photocatalyst: application for degradation of 4-cholorophenol. Journal of Hazardous Materials, 252-253, 233–242.
Pandikumar, A., & Ramaraj, R. (2012). Titanium dioxide–gold nanocomposite materials embedded in silicate sol–gel film catalyst for simultaneous photodegradation of hexavalent chromium and methylene blue. Journal of Hazardous Materials, 203-204, 244–250.
Pearce, C. Lloyd, J. R., & Guthrie, J.T. (2003). The Removal of Colour From Textile Wastewater Using Whole Bacterial Cells: A Review. Dyes and Pigments, 58(3), 179–196.
Pugazhenthiran, N., Murugesan, S., & Anandan, S. (2013). High surface area Ag-TiO2 nanotubes for solar/visible-light photocatalytic degradation of ceftiofur sodium. Journal of Hazardous Materials, 263, 541–549.
Punzi, M., Anbalagan, A., Börner, R. A., Svensson, B.-M., Jonstrup, M., & Mattiasson, B. (2015). Degradation of a textile azo dye using biological treatment followed by photo-Fenton oxidation: Evaluation of toxicity and microbial community structure. Chemical Engineering Journal, 270, 290–299.
Qin, X., Jin, L., Tian, G., Qu, Y., & Feng, Y. (2009). Enhanced photocatalytic activity for degrading Rhodamine B solution of commercial Degussa P25 TiO2 and its mechanisms. Journal of Hazardous Materials, 172(2-3), 1168–1174.
Ramos-delgado, N. A., Gracia-pinila, M., Maya-treviño, L., Reyes, H., Guzman-mar, L. J., & Hernández-Ramírez, L. A. (2013). Solar photocatalytic activity of TiO2 modified with WO3 on the degradation of an organophosphorus pesticide. Journal of Hazardous Materials, 263(1), 36–44.
Rasalingam, S., Peng, R., & Koodali, R. T. (2015). An insight into the adsorption and photocatalytic degradation of rhodamine B in periodic mesoporous materials. Applied Catalysis B: Environmental, 174-175, 49–59.
Rehman, S., Ullah, R., Butt, A. M., & Gohar, N. D. (2009). Strategies of making TiO2 and ZnO visible light active Journal of Hazardous Materials, 170(2-3), 560–569.
Robinson, T., Mcmullan, G., Marchant, R., & Nigam, P. (2001). Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresource Technology, 77, 247–255.
Rochkind, M., Pasternak, S., & Paz, Y. (2014). Using dyes for evaluating photocatalytic properties: a critical review. Molecules, 20(1), 88–110.
Semeraro, P., Rizz, V., Fini, P., Matera, S., Cosma, P., Franco, E., García, R., Ferrándiz, M., Núñez, E., Gabaldón, J. A., Fortea, I., Pérez, E., & Ferrándiz, M. (2015). Interaction between industrial textile dyes and cyclodextrins. Dyes and Pigments, 119, 84–94.
Spasiano, D., Marotta, R., Malato, S., Fernandez-Ibanezand, P., & Somma, I. D. (2015). Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Applied Catalysis B: Environmental, 170, 90–123. https://doi.org/10.1016/j.apcatb.2014.12.050.
Wang, J., Jing, L., Xue, L., Qu, Y., & Fu, H. (2008). Enhanced activity of bismuth-compounded TiO2 nanoparticles for photocatalytically degrading rhodamine B solution. Journal of Hazardous Materials, 160, 208–212.
Yu, K., Yang, S., He, H., Sun, C., Gu, C., & Ju, Y. (2009). Visible light-driven photocatalytic degradation of rhodamine B over NaBiO3: pathways and mechanism. The Journal of Physical Chemistry. A, 113, 10024–10032.
Yuan, R., Zhou, B., Hua, D., & Shi, C. (2013). Enhanced photocatalytic degradation of humic acids using Al and Fe co-doped TiO2 nanotubes under UV/ozonation for drinking water purification. Journal of Hazardous Materials, 262, 527–538.
Funding
The authors received financial support from the following Brazilian institutions: PROPP/UFOP, Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG-Grants APQ-00847-14), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oliveira, V.L., Lima, A.L.D., Gabriel, J.B. et al. Enhanced Photocatalytic Activity of TiO2/γ-Fe2O3 by Using H2O2 as an Electron Acceptor Under Visible Light Radiation. Water Air Soil Pollut 231, 575 (2020). https://doi.org/10.1007/s11270-020-04916-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04916-0