Abstract
The use of non-invasively collected DNA source material for genetic and genomic applications is usually characterized by low target DNA concentration and quality, genotyping errors and cost-intensive lab procedures. However, for otters (Lutrinae) as elusive species of conservation concern, genetic non-invasive sampling has become an important tool to study their ecology and demography. To increase cost-efficiency of monitoring programmes and to promote the expansion of genomic approaches to non-invasive samples, we aimed to refine sample collection and preparation. Therefore, we examined the effects of intrinsic sample characteristics (including diet), environmental conditions in the field and sample treatment in the molecular laboratory on the success of genotyping and allelic dropout (ADO) rates using microsatellite markers in 1970 fresh Eurasian otter (Lutra lutra) scats. Using fresh samples only, we probably eliminated one of the most important impediments of genotyping DNA from otter faecal samples beforehand. But, we observed higher genotyping success and lower ADO rates for anal glad secretions and faecal samples containing high proportions of mucus. Moist conditions during sample collection may promote DNA degradation and PCR inhibition, leading to decreased genotyping success rates. ADO was further affected by the type of extraction kit. However, a high proportion of variance remaining unexplained by our models implied that additional parameters were acting (amount of PCR inhibitors, non-uniform distribution of intestinal cells, efficiency of PCRs, specific microclimate at marking sites). We summarized influential factors maximizing genotyping quality of otter scats and give recommendations for sample collection, storage and DNA extraction based on our results and current literature.
Similar content being viewed by others
Introduction
The use of non-invasive genetic methods in wildlife research and management has gained on importance due to continuous advances in molecular genetic techniques (Schwartz et al. 2007; Beja-Pereira et al. 2009; De Barba et al. 2017; Andrews et al. 2018; Carroll et al. 2018). Especially for species of conservation concern and for elusive and nocturnal species, genetic non-invasive sampling facilitates their investigation without the need of disturbing, capturing and handling the animals (Waits and Paetkau 2005). Within the field of non-invasive genetic monitoring, faecal samples have been widely used as DNA from sloughed epithelial cells of the intestine wall of the defecating individual can be analysed (Kohn and Wayne 1997). Faeces are constantly produced by every animal in a population, and for many animal species, they are abundant in the field and relatively easy to detect. Another advantage is that sampling permissions are not required (Beja-Pereira et al. 2009).
Despite of the seductive advantages using non-invasive samples, there are certain limitations when using them as source of DNA. In particular, extracted target DNA from faeces is usually degraded, available in very low amounts, and finally, bacteria and enzymes may be present in considerable amounts (Taberlet et al. 1999). The latter is a common problem with faecal samples as such enzymes and bacteria may act as inhibitors in polymerase chain reactions (PCRs) in subsequent analyses.
These factors often may lead to erroneous genotypes and/or reduce the genotyping success and hence the effective sample size (Taberlet and Luikart 1999). Genotyping errors are primarily due to allelic dropouts (ADOs) and false alleles (Broquet and Petit 2004; Pompanon et al. 2005), which can lead to the creation of so-called ghost individuals or an inability to distinguish individuals (Taberlet et al. 1999; Taberlet and Luikart 1999). As genotyping data are often used in population studies, such errors could cause false results, e.g. a bias in abundance estimates (Lampa et al. 2015a). Therefore, rigorous standards for the analysis of non-invasive samples have been established to minimize genotyping errors (Navidi et al. 1992; Taberlet et al. 1996; Morin et al. 2001; Beja-Pereira et al. 2009; Hausknecht et al. 2010). However, this is at the cost of increased personnel and material expenses (De Barba and Waits 2010). Moreover, high sample dropout rates produce high costs, as more samples have to be collected and analysed to obtain an adequate sample size.
Besides traditional sequencing methods using genetic markers (e.g. microsatellites), also next-generation sequencing methods (e.g. RAD sequencing, DNA metabarcoding) depend on non-invasively collected sample material when dealing with elusive and protected animals to identify the consumer species and individual, and its diet (Monterroso et al. 2019). However, genomic approaches require large amounts of high-quality template DNA (Carroll et al. 2018), and generally, analysis costs for next-generation sequencing are still too high to allow high sample dropout rates due to PCR inhibition or low DNA concentrations. Thus, there is a need to optimize sample collection and DNA extraction of samples collected non-invasively to reduce negative samples and genotyping errors and increase the DNA yield.
DNA quality and quantity isolated from faeces may be influenced by multiple factors in the field, in the subsequent sample storage and lab procedures (Murphy et al. 2003; Piggott and Taylor 2003; Waits and Paetkau 2005; Murphy et al. 2007; Broquet et al. 2007). Several studies on different species and regions have already identified various factors influencing DNA quality including environmental conditions (temperature, humidity, sun exposure) (e.g. Nsubuga et al. 2004; Murphy et al. 2007; Agetsuma-Yanagihara et al. 2017), sample preservation method (e.g. Frantzen et al. 1998; Santini et al. 2007; Bubb et al. 2011), DNA extraction method (e.g. Hájková et al. 2006), storage duration until extraction of DNA (e.g. Soto-Calderón et al. 2009; Bourgeois et al. 2019) and diet of the defecating animal (Murphy et al. 2003; Panasci et al. 2011). Broad consensus was found regarding the age of the scat samples: genotyping success rates and sample quality were highest using the freshest samples (Piggott 2004; Murphy et al. 2007; Brinkman et al. 2010; Vynne et al. 2012; Bourgeois et al. 2019). However, since texture of faeces, diet and environmental conditions may differ between species and populations, influencing factors can vary with different animal species and even within one species occurring in different habitats (Panasci et al. 2011). Generally, carnivores seem to be the most challenging group regarding genetic analysis of faecal material (Ramón-Laca et al. 2015). Within the group of carnivores, fish-eating species seem to be the most problematic (Reed et al. 1997; Murphy et al. 2003). Hence, it is not surprising that the genetic analysis of otter (Lutrinae) faeces is deemed as notoriously tricky (Lerone et al. 2014; Klütsch and Thomas 2018) and characterized by high dropout rates (about 50%; e.g. Hájková et al. 2009; Mowry et al. 2011; Silveira Trinca et al. 2013; Vergara et al. 2014; Sittenthaler et al. 2015; Biffi and Williams 2017; Sittenthaler et al. 2020). Nevertheless, when studying otter species, faeces are the sample material of choice, as otters are elusive living animals depositing scats (so-called spraints) regularly at conspicuous marking sites close to the water body (Kruuk 2006). All but one (Lontra canadensis) of the 13 otter species worldwide are of conservation concern (IUCN 2020), and non-invasive genetic sampling methods have become an important research tool, which has been integrated in a large number of otter monitoring schemes.
Previous studies investigating genotyping success, amplification rate and error rate of faecal samples in otters have tested a variety of different storage media and extraction kits (Fike et al. 2004; Hájková et al. 2006; Lampa et al. 2008; Lerone et al. 2014). Some of these studies also considered the effect of sample type, sample age, storage time, temperature and humidity during collection on genotyping quality, yielding the best results for fresh samples (Jansman et al. 2001; Hájková et al. 2006; Lerone et al. 2014) extracted soon after collection (Lampa et al. 2008). Many studies showed that slimy secretions of anal scent glands of otters (so-called anal jellies) outperformed typical otter faeces (Coxon et al. 1999; Fike et al. 2004; Hájková et al. 2006; Lampa et al. 2008; Mowry et al. 2011; Lerone et al. 2014). Focusing on the influence of temperature and humidity during collection of the samples, some studies led to contradicting results (Hájková et al. 2006; Lerone et al. 2014; Martin et al. 2017). However, most of these studies were restricted by low sample sizes and/or could include only a limited number of variables in their statistical analyses. Additionally, none of the studies investigated the effect of diet (e.g. crayfish-rich diet) on DNA quality. In the present study, we used a large data set consisting of 1970 faecal Eurasian otter (Lutra lutra), hereinafter referred to as otter, samples collected in temperate Central Europe and analysed the effects of a set of variables on nuclear DNA (microsatellite) genotyping success and genotyping error rate (allelic dropout), both representing quantity and quality of target DNA. The variables included (i) intrinsic sample characteristics (diet composition, sample type, sex of the otter individual), (ii) environmental conditions in the field during sample collection (ambient temperature, humidity, season) and (iii) sample treatment in the molecular laboratory (sample storage time until DNA extraction, type of extraction kit). Although we did not apply genomic approaches, but focused on marker-based analysis, our study aimed to refine and optimize the collection and preparation of non-invasive samples for both traditional and modern genetic approaches.
Material and methods
Faecal sample collection and preservation
We collected otter scats in seven different study sites (one pond area, six salmonid streams) located in northern and eastern parts of Austria (Table 1; Online Resource 1: Fig. S1). Initially, we defined sampling sites along the river and pond banks, representing typical otter marking sites. As the age of a scat has already been identified as crucial factor for DNA degradation and hence genotyping success (Nsubuga et al. 2004; Hájková et al. 2006; Lerone et al. 2014), we used only otter scats deposited within the last 24 h before collection. Hence, we removed all old otter scats from a sampling site and inspected every site repeatedly on three to six consecutive days to ensure a collection of fresh samples only.
Otter scats appeared in various shapes and consistencies, mainly differing by the amount of mucus, and hence potentially the number of excreted cells of the intestinal mucosa. We classified them into three distinct types: spraints (consisting of prey remains and almost no mucus), jellyspraints (consisting mainly of mucus and to a lesser extent of prey remains) and anal jellies (slimy secretion of anal scent glands with hardly any prey remains). All samples were stored in a sample buffer (for details, see Sittenthaler et al. 2015) at minus 20 °C until DNA extraction. The time span between collection and DNA extraction of the sample was defined as storage time.
Climate data and seasons
According to the collection date, samples were assigned to meteorological seasons: spring (March–May), summer (June–August), autumn (September–November) and winter (December–February). In addition, for each study area and sampling year, data on ambient temperature and relative humidity of the closest meteorological station were provided by Zentralanstalt für Meteorologie und Geodynamik (www.zamg.ac.at), Amt der Niederösterreichischen Landesregierung (www.noe.gv.at) and Höhere Lehranstalt für Umwelt und Wirtschaft Yspertal (www.hluwyspertal.ac.at). The combination of high temperatures and humid conditions lead to higher enzyme and bacteria activity in a faecal sample and promote DNA degradation (Kohn et al. 1995). To account for this effect, we included hourly measured maximum values of ambient temperature (°C) and relative humidity (%) in our analysis. The climate data covered the time frame between dusk the day before collection (when otters start to get active, Kruuk 2006) and 2 p.m. on the day of sample collection (when field work was finished).
DNA extraction and genotyping of otter scats
Faecal DNA was extracted using two different DNA extraction kits, the standard QIAamp DNA Stool Mini Kit and the redeveloped Fast QIAamp DNA Stool Mini Kit (both Qiagen, Germany) following the manufacturer´s protocol with slight modifications, i.e. an increase of final incubation time to 5 min before elution of the DNA and elution of the DNA with 100 μl AE buffer (Qiagen, Germany). Samples were genotyped at 11 (dinucleotide and tetranucleotide) microsatellite loci established by Dallas and Piertney (1998): Lut435, Lut457, Lut615, Lut701, Lut717, and Lut833 (multiplex set 1) and Lut453, Lut604, Lut715, Lut733, and Lut832 (multiplex set 2), using the protocol and PCR conditions described in Sittenthaler et al. (2015). For characteristics of microsatellites used in this study, we refer to Online Resource 1: Table S1. Fragment length analysis was performed on an ABI PRISM® 377 DNA sequencer (Perkin Elmer, Germany) and an ABI PRISM® 3130xl Sequence Analyser (Applied Biosystems, USA). Alleles were analysed using automatic allele recognition and manually reviewed with GENESCAN 3.1.2, GENOTYPER 2.5 and GENEMAPPER 5.0 software (Applied Biosystems, USA).
Initially, all samples were amplified in independent quadruplicates for the first multiplex set to identify samples which failed to amplify in all of the six loci. Subsequently, remaining samples, i.e. samples that showed amplification for multiplex set 1, were processed with marker set 2 and up to eight additional PCR reactions for both sets were performed to determine a sample´s consensus genotype (referring to the multiple tube approach by Taberlet et al. 1996), according to the following rules: a heterozygote genotype was accepted when each of both alleles was recorded at least twice and a homozygote genotype after at least three independent replications of a single allele. Sex identification was performed as described in Sittenthaler et al. (2015), also in independent quadruplicates. Meeting the standards of working with non-invasive DNA samples, negative controls were included throughout the extraction procedure and PCR amplifications to check for possible contaminations. Furthermore, pre- and post-PCR pipetting was carried out in different laboratory rooms.
After laboratory analysis, we evaluated the genotyping success for every sample (positive/negative). We counted a sample as positive when at least nine of 11 microsatellites (adequate number of loci to differentiate even between closely related individuals according to Sittenthaler et al. 2020) could be scored unambiguously in the consensus genotype. We further calculated genotyping error rates (ADO) for every positive sample across loci based on the consensus genotype obtained from repeated genotyping and multiple recaptures of the same otter individuals. ADO rate was quantified as the number of allelic dropouts over the total number of successful amplifications of heterozygote loci for a given sample according to Broquet and Petit (2004).
Otter diet composition
For a subset of our samples (N = 714; study areas Ysper, Piesting and Ois), we analysed hard prey remains in otter scats (for details of dietary analysis of otter faeces, see Sittenthaler et al. 2019). This subset consisted only of spraints and jellyspraints, as anal jellies did not contain any prey remains. We performed a hierarchical cluster analysis to classify the samples in different diet categories, based on presence/absence data of hard prey remains of the main prey categories (fish, crayfish, amphibians, birds, reptiles, mammals, insects). We used the R package cluster (Maechler et al. 2019), and applied the Ward clustering algorithm (Ward 1963).
Statistical analysis and modelling
To analyse the effects of sample characteristics, environmental conditions in the field and sample treatment in the molecular analysis on genotyping success and allelic dropout rate of a sample, we set up four different models. As our response variables were either binary (genotyping success: positive/negative) or proportional (allelic dropout rate 0–100%) and due to the fact that otter scats were repeatedly collected at marking sites from the same individuals, we applied generalized linear mixed models (GLMMs) with a binomial distribution and logit link function.
In our first model, we fitted a GLMM with genotyping success (positive and negative samples, N = 1970) as response variable. We included the following fixed covariates: season, sample type, extraction kit, storage time, temperature and humidity, as well as an interaction term between the latter two. The sample location, i.e. marking site, was included as a random intercept.
For our second model, we used the same model parameters as in model 1, but reduced the data set (positive and negative samples, N = 714) to include diet as an additional fixed explanatory variable. Additionally, we had to exclude the covariate extraction kit, as within the reduced data set, we only used one DNA extraction kit.
In the third model, we fitted a GLMM for all positive samples (N = 1049) with the proportion of allelic dropout (0–100%) per sample as response variable. As fixed covariates, we used season, extraction kit, sample type and sex of the corresponding otter individual. As according to Lampa et al. (2015b), male otters are supposed to leave more anal jellies than females, we included an interaction term between the variables sample type and sex. Further, we included the fixed covariates storage time, temperature and humidity (and again an interaction term between the latter two). In addition to sample location, we also included the otter individual as random intercept.
In our fourth model, we principally used the same model parameters as in model 3, but again used a reduced data set, including only those positive samples for which we had analysed diet composition (N = 340). Consequently, in model 4, we used diet as additional fixed factor and again had to exclude the covariate extraction kit (see model 2).
Before fitting the four models, we followed the protocol by Zuur et al. (2010) for data exploration to check specifically for outliers in the data sets, multicollinearity of the explanatory variables and independence of the response variable. There was no multicollinearity between variables; further, we found no indications for the inclusion of more interactions than the ones we mentioned above. We used the R package lme4 (Bates et al. 2015) to generate a global model for each response variable including all parameters. To identify important explanatory variables, we applied a multimodel inference approach (Burnham and Anderson 2002; Burnham et al. 2011) using the R package MuMIn (Bartón 2018). For a set of models representing all possible combinations of explanatory variables, we calculated the Akaike information criterion (AICc) (Hurvich and Tsai 1989), and finally selected those models with a ΔAICc < 2 (Burnham et al. 2011). Model-averaged coefficients were calculated based on the subset of models, and conditional averaging was used (Grueber et al. 2011; Symonds and Moussalli 2011). Further, for each parameter of the final model, we calculated relative importance values and corresponding upper and lower bounds of the 95% confidence intervals (CIs). Model parameters not including zero within their CI were considered to be informative (Grueber et al. 2011). The amount of variance explained by the models is presented as marginal and conditional R2 for GLMMs (Nakagawa and Schielzeth 2013). All statistical analyses were performed using the software R 3.5.1 (R Core Team 2018).
Results
We included a total of 1970 otter faecal samples (1401 spraints, 407 jellyspraints, 162 jellies) in our analysis, of which 1049 revealed a positive genotyping result (overall genotyping success rate of 53%). ADO rates across all loci were not normally distributed (Shapiro-Wilk normality test: W = 0.872, p < 0.001), and the overall median was 17.2% with a minimum value of 0.0 and a maximum of 96.9% (Table 2). For a total of 714 samples, diet analysis was conducted, while the number of positive samples including information on diet totalled 340 (Table 2). Hierarchical cluster analysis revealed a classification of otter samples into 5 diet categories: (1) samples containing amphibian bones, N = 79; (2) samples containing bird remains, N = 75; (3) samples consisting of fish remains only, N = 432; (4) samples containing mammalian and crayfish remains, N = 58; and (5) samples containing mainly crayfish, N = 70. Maximum ambient temperature at sampling dates ranged between − 4.4 and 32.0 °C (mean ± SD 12.9 ± 7.9 °C), maximum relative humidity between 56 and 100% (mean ± SD 92.1 ± 9.4%) and storage time covered the range between 0 and 28 days (mean ± SD 3.8 ± 1.9 days).
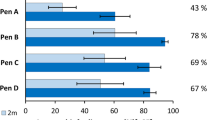
For modelling the genotyping success (model 1), the subset of models with a ΔAICc < 2 consisted of nine models (Table 3). The final averaged model included the variables sample type, extraction kit, storage time, season, temperature and humidity. However, the confidence intervals for the parameter estimates of extraction kit, storage time, temperature and humidity included zero and importance values that were below 0.5, indicating little evidence that these predictors affected genotyping success of otter samples (Table 4). With an importance value of 1, sample type had a significant effect on genotyping success. We found lowest genotyping success rates for spraints and highest rates for jelly samples (Fig. 1a, Table 4). Coefficients from the final model showed that using spraints or jellyspraints instead of jelly samples for genotyping, the odds for a positive result decreased by 15 and 27%, respectively (Table 4). Although we detected higher genotyping success rates in summer compared with autumn (Table 4), an importance value of 0.09 indicated little evidence that the variable season actually affected genotyping success.
For our second model analysing genotyping success with the reduced data set, the subset of models comprised only two models, including the variables humidity, sample type and storage time, whereas the latter had no effect (Tables 3 and 4). With an increase of 10% in maximal humidity, the odds of a positive genotyping result decreased by 82%. Also in the reduced data set, sample type affected genotyping success: the odds of obtaining a positive genotyping result using spraints as source material decrease by 17% and 44% compared with jelly samples and jellyspraints, respectively (Table 4).
Modelling proportion of ADO rate including all positive samples (model 3), the multimodel inference approach revealed a model subset consisting of four models (Table 3). The variables extraction kit, sample type, sex, an interaction of the latter two, storage time and temperature were included in the final averaged model. The confidence intervals for the parameter estimates of sex, storage time, temperature and the interaction term included zero, while extraction kit and sample type affected ADO rate, also indicated by the importance values of 1 (Table 4). Compared with the redeveloped Fast QIAamp DNA Stool Mini Kit, lower allelic dropout rates were generated with the standard stool extraction kit (Table 4). Regarding sample type, higher ADO rates were produced by using jellyspraint and spraints samples, with odds increasing 2.6 times and 3.8 times compared with anal jellies (Fig. 1b, Table 4).
For model 4 (modelling proportion of ADO rate with the reduced data set), we obtained a subset consisting of six models (Table 3). Here, seven variables were included in the final model, but only the variables diet, season and temperature had a confidence interval not including zero. Nevertheless, the low importance values (0.29) of these model parameters indicated little evidence that these predictors affected ADO rate much.
For all models, variance explained as shown by R2GLMM(m) and R2GLMM(c) ranged between 0.06 and 0.14 and indicated little variance explained by the final models.
Discussion
In the present study, we examined factors impacting microsatellite genotyping success and allelic dropout rates of otter scats collected in temperate Central Europe. Of the tested variables, only few had a considerable influence on these two parameters indicating the quality of the DNA. Most studies on genotyping animal scats collected in the field highlighted the importance of using fresh scats to increase DNA quality (e.g. Murphy et al. 2007; Vynne et al. 2012; Lerone et al. 2014; Bourgeois et al. 2019). In the present study, we have already considered this factor and collected only otter faeces from the previous night. Thereby, we probably eliminated one of the most important impediments for genotyping DNA from otter faecal samples beforehand. We showed that genotyping success rate depended mostly on the type of sample and humidity during sample collection, whereas the latter was only significant in the reduced data set (model 2). Allelic dropout was mainly affected by sample type and extraction kit. Using the reduced data set, none of the considered variables was of high relevance explaining ADO rate.
In accordance with previous studies on different otter species using scats for genetic analyses, we observed significantly higher genotyping success rates and to a lesser extent lower allelic dropout rates for anal jelly samples (Fike et al. 2004; Hájková et al. 2006; Lampa et al. 2008; Mowry et al. 2011; Silveira Trinca et al. 2013; Lerone et al. 2014; Biffi and Williams 2017). Furthermore, as already pointed out by Hájková et al. (2006) and Lerone et al. (2014), not only when using anal jellies, but also otter scats containing high proportions of mucus (jellyspraints) led to significant better genotyping results compared with otter scats mainly consisting of prey remains and less mucus.
Concerning climate conditions during sample collection, similar studies investigating DNA quality of scats originating from various animal species and regions concluded that either dry and/or very cold climatic conditions preserve the DNA in the field best (Piggott 2004; Murphy et al. 2007; Brinkman et al. 2010; Demay et al. 2013; Agetsuma-Yanagihara et al. 2017; Nakamura et al. 2017; Klütsch and Thomas 2018). With otter scats deposited in the night before collection, our results indicated that ambient temperature seemed to have no impact on DNA quality, whereas the probability of a sample resulting in a positive genotype decreased with increasing humidity. However, this effect was not constantly significant in all our models. Although Hájková et al. (2006) found that PCR amplification success of otter scat samples was higher at colder temperatures, the overall effect of temperature was weak compared with the impact of other factors (e.g. sample type). Contradictory to their findings, Lerone et al. (2014) observed higher genotyping success rates with increasing mean daily temperatures, but they assumed that the observed temperature effect was confounded with other undetected influential environmental factors. However, they also found lower PCR success rates with increasing humidity, which was confirmed by the results of the present study. Summarizing, ambient temperature during the collection of otter scats for genetic analyses may play a tangential role when sample collection takes place within 24 h after scat deposition, while moist conditions seem to offer potential to high enzyme and bacteria activity resulting in DNA degradation and PCR inhibition (Kohn et al. 1995; Lampa et al. 2008).
The DNA extraction kits we used in this study were specifically designed for extracting DNA from stool samples; hence, both of them include procedures to remove some of the enzymes and bacteria present in faeces, which can act as PCR inhibitors in downstream analyses. As there was no effect of extraction kit on genotyping success, we conclude that both kits removed PCR inhibitors to a comparable level. However, based on our results, the standard kit with the InhibiteEX Tablets (Qiagen, Germany) performed slightly better concerning DNA quality, as ADO rates were significantly lower (Table 4). To our knowledge, in our field of application, there does not exist another study comparing these two kits. However, other studies comparing the efficiency of various DNA extraction protocols for extracting DNA of faecal material reported higher DNA yield and genotyping success for faecal samples extracted with commercially available kits specifically designed for stool samples (Bhagavatula and Singh 2006; Lampa et al. 2008; Beja-Pereira et al. 2009). A reduction of genotyping errors leads to a reduction of material and personnel costs, as efforts to obtain a reliable genotype decrease. Hence, to ensure a high level of genotyping success and low error rates, we recommend to use DNA extraction kits designed to remove PCR inhibitors in faecal samples.
In accordance with most previous studies, the time span between sample collection and DNA extraction neither had an effect on genotyping success, nor on ADO rate (Hájková et al. 2006; Lerone et al. 2014). However, Lampa et al. (2008) reported a significant decrease in amplification success rate by increasing storage time with highest declines after 1 week. Studying the effects of storage type and time on DNA amplification success in tropical ungulate faeces, Soto-Calderón et al. (2009) also recommended to extract the DNA within 1 week after collection to ensure optimal recovery of DNA. As the effect of storage time may also depend on how samples have been preserved, these contradictory results may have been arisen due to the usage of different sample storage media. Otter faecal DNA samples stored in a buffer solution or ethanol (Hájková et al. 2006; Lerone et al. 2014) seemed to be preserved over a longer time span compared with samples deep-frozen without a special storage buffer (Lampa et al. 2008). This assumption is supported by our samples, which have been stored at minus 20 °C in a self-made buffer solution comparable with the RNAlater® stabilization reagent, which is considered to be one of the best preservation techniques for faecal sample material over longer storage periods (Soto-Calderón et al. 2009; Beja-Pereira et al. 2009). As all studies specifically testing the effect of storage time covered not more than a few months of storage, future studies should investigate if and how long the DNA will still be sufficiently preserved after sample collection. In a study on otter population dynamics, Bonesi et al. (2013) extracted the DNA of otter scat samples stored in ethanol 2 years after sample collection and yielded a relatively low genotyping success rate of only 19%. However, apart from long storage periods, the authors discussed several factors that may have contributed to the poor result (namely, sample collection during warm periods, wrong sample preservation and few anal jellies among the analysed samples).
We assumed that different diets containing varying proportions of undigestible dietary components will differ in their digestion time and in their intestinal cell slough rates and therefore influence the amount of abraded cells containing target DNA in a faecal sample (Murphy et al. 2003). In the present study, this variable was included for the first time in comparable analyses on genotyping quality of otter faeces. However, based on our results, different prey types (e.g. crayfish containing high proportion of undigestible dietary components) consumed by otters in stream and pond habitats in temperate Central Europe did not impact genotyping success. Although there was a significant effect of diet on ADO rate, relative importance was very low, indicating a very weak effect on DNA quality.
The generally low genotyping success rates in otters compared with other mammal species may be caused by their diet, which is usually based on fish (Kruuk 2006; Krawczyk et al. 2016). Murphy et al. (2003) reported significantly lower DNA amplification success rates in bears when they were fed on salmon. They assumed that the high lipid content and the low dietary fibre content of fish may lead to less abrasion of intestinal cells that contain target DNA or that there may be an interference with extraction chemicals. The latter might explain why different prey types did not impact genotyping quality, as in a free-ranging otter´s digestive system there will be always a certain proportion of fish and its chemical components potentially hampering efficiency of DNA extraction or promoting DNA degradation.
We included the sex of the otter individuals and the interaction with sample type in our models to take into account that a certain sample type and its DNA quality is linked to either males or females. While Lampa et al. (2015b) found that male otters leave more anal jellies than females, we did not observe a sex difference in deposition rate of sample types, and consequently we did not observe any sex bias due to higher genotyping success rates of anal jellies and jellyspraints compared with spraints.
Due to a large data set, we were able to include a large number of parameters that potentially affect the quality of genotyping otter scats. However, it is important to note that our models explained only a small amount of variance of genotyping success and ADO rates (Table 3). Unfortunately, it is not possible to draw comparisons with results of previously published studies, since most of them did not report values for the goodness of fit of their models. However, the high proportion of variance remaining unexplained by our models implies that additional parameters were acting, which were not included in our models because they were difficult or even impossible to quantify. One factor that could have potentially affected genotyping success and ADO rate is a non-uniform distribution of intestinal cells in our samples. A successful extraction of DNA molecules may be depended on the location on the scat where the sample for extraction was taken. The impact of sampling location within a faeces on DNA quality of two carnivores was shown by Stenglein et al. (2010), who reported significantly higher genotyping quality when taking samples from the tip (grey wolf, Canis lupus) and the inside (brown bear, Ursus arctos) of the scat. Compared with other carnivore scats, it is not always possible to distinguish between front end, back end and middle of an otter scat, especially when the proportion of mucus is high making its shape formless. An adoption of the extraction protocol in order to use larger volumes of initial faecal material may help to increase the chance of capturing enough intestinal cells for DNA extraction (Sittenthaler et al. 2018). Alternatively, conducting more than one extraction per sample was suggested by Frantzen et al. (1998) and Nsubuga et al. (2004), though leading to an extra time and financial effort.
Another factor, which is difficult to identify and measure, is the presence and amount of PCR inhibitors related to diet consumed or the underground where the scat was deposited by the animal. Some authors suggested swabbing protocols instead of collecting the whole scat sample in order to optimize target DNA recovery and limit the presence of PCR inhibitors to a minimum (Klütsch and Thomas 2018; Bourgeois et al. 2019). However, other studies found no differences in amplification success and genotyping error rate between the different sampling protocols (Velli et al. 2019) or even better results with the traditional approach (Miles et al. 2015). Lampa et al. (2008, 2015b) applied a swabbing protocol to otter samples, but yielded similar genotyping success rates like others collecting the whole scat (e.g. this study; Hájková et al. 2009; Vergara et al. 2014). Martin et al. (2017) used swabs and yielded an overall success rate of only 23%, which is about the half compared with the others.
Moreover, also factors like the efficiency and specificity with which PCR primers anneal or other PCR conditions, as well as secondary structures in template DNA might influence amplification success and genotyping error rates.
Although it is commonly assumed for the genetic analysis of non-invasive samples that longer fragments show lower amplification success rates and higher error rates (e.g. Sefc et al. 2003; Buchan et al. 2005; Broquet et al. 2007), this was not the case with our microsatellite markers selected for this study (see Table S1 in Online Resource 1; Hájková et al. 2009; Hausknecht et al. 2010; Sittenthaler et al. 2015). Also, Lerone et al. (2014) found no relationship between molecular weight and genotyping error rate, but a decrease in PCR success rate with increased median allele size.
In order to correctly analyse the impact of temperature and humidity, it would have been beneficial to measure the specific microclimate at each sampling point rather than using climate data of meteorological stations for the whole study area. Especially along water bodies there might be large differences in microclimate on a very small scale. The same may be valid for UV light exposure of the scat sample. Another reason why we were not able to detect a temperature effect could be the lack of knowledge on the exact time period for how long a deposited scat was exposed to the environmental conditions. However, measuring meteorological parameters at marking sites (especially, on stones with sun exposure) is almost impossible. Therefore, we suggest ex situ experiments under controlled conditions in a lab, which would allow to adjust parameters and simulate field scenarios. Such experiments may shed light on the influence of climatic conditions on genotyping quality of otter faeces.
Despite of the relatively low genotyping success rates of faecal otter samples (about 50%; Lanszki et al. 2008; Janssens et al. 2008; Hájková et al. 2009; Bonesi et al. 2013; Vergara et al. 2014; Lerone et al. 2014; Sittenthaler et al. 2015; Martin et al. 2017) compared with faeces of other semi-aquatic mammals (e.g. 67% Pyrenean desman, Galemys pyrenaicus (Gillet et al. 2017); 89% American mink, Neovison vison (Velando et al. 2017)) and other animal species (e.g. 96% Black grouse, Tetrao tetrix (Sittenthaler et al. 2018); 66% brown bear Ursus arctos (Frosch et al. 2014)), the usefulness of otter spraints as non-invasive source of DNA for genetic and population demography analyses is unquestioned. However, it seems that one has to accept and allow for sample dropout rates of about 50%, which may be related to factors difficult or impossible to control. In the light of increasing the cost-efficiency of DNA-based conservation studies and monitoring programmes (e.g. Ferreira et al. 2018), as well as enabling the application of non-invasive genetic methods in the expanding field of genomics, we here summarize influential factors maximizing genotyping quality of otter scats and give recommendations for sample collection, storage and DNA extraction:
-
1.
Samples collected should be as fresh as possible, i.e. maximum 24-h-old samples, to limit DNA degradation.
-
2.
Sample design has to account for high dropout rates (about 50%) regarding sample sizes. Thus, multiple samples, at least twice as many samples as needed for respective statistics, should be taken from the same location to maximize the chances to detect all individuals present.
-
3.
Even if anal jellies provide the most promising genotyping results, one should not only focus on the collection of anal jellies. Firstly, they are available in the field to a lesser amount than other sample types, and secondly, they pose potential risk of a certain male bias (Lampa et al. 2015b). In addition to jelly samples, spraints containing a high proportion of mucus (jellyspraints) also showed above-average genotyping success rates.
-
4.
Although there are contradictory results concerning temperature and humidity during sampling, we recommend to plan sample collection during dry periods. As long as fresh samples are taken only, it does not matter if sampling takes place during warm or cold seasons—at least in temperate climatic zones as it was the case of the current study.
-
5.
Use commercially available DNA extraction kits specifically designed to remove PCR inhibitors which may be present in faecal samples.
-
6.
To be on the safe side, storage times (i.e. the time span from collection to DNA extraction of a sample) should be as short as possible, not exceeding 1 week. However, when using a buffer solution, length of storage time may not be of relevance, at least as samples will be extracted within a few months.
-
7.
Allow for more extractions per sample, if necessary. Further, the use of larger volumes of stool material in DNA extraction may maximize the chances to capture enough sloughed cells and hence DNA.
References
Agetsuma-Yanagihara Y, Inoue E, Agetsuma N (2017) Effects of time and environmental conditions on the quality of DNA extracted from fecal samples for genotyping of wild deer in a warm temperate broad-leaved forest. Mammal Res 62:201–207
Andrews KR, De Barba M, Russello MA, Waits LP (2018) Advances in using non-invasive, archival, and environmental samples for population genomic studies. In: Population Genomics. Springer, Cham. https://doi.org/10.1007/13836_2018_45
Bartón K (2018) MuMIn: Multi-Model Inference. R package version 1(42):1 https://CRAN.R-project.org/package=MuMIn.
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Beja-Pereira A, Oliveira R, Alves PC et al (2009) Advancing ecological understandings through technological transformations in noninvasive genetics. Mol Ecol Resour 9:1279–1301
Bhagavatula J, Singh L (2006) Genotyping faecal samples of Bengal tiger Panthera tigris tigris for population estimation: A pilot study. BMC Genet 7:48
Biffi D, Williams DA (2017) Use of non-invasive techniques to determine population size of the marine otter in two regions of Peru. Mamm Biol 84:12–19
Bonesi L, Hale M, Macdonald DW (2013) Lessons from the use of non-invasive genetic sampling as a way to estimate Eurasian otter population size and sex ratio. Acta Theriol 58:157–168
Bourgeois S, Kaden J, Senn H, Bunnefeld N, Jeffery KJ, Akomo-Okoue EF, Ogden R, McEwing R (2019) Improving cost-efficiency of faecal genotyping: New tools for elephant species. PLoS One 14(1):e0210811
Brinkman TJ, Schwartz MK, Person DK, Pilgrim KL, Hundertmark KJ (2010) Effects of time and rainfall on PCR success using DNA extracted from deer fecal pellets. Conserv Genet 11:1547–1552
Broquet T, Ménard N, Petit E (2007) Noninvasive population genetics: a review of sample source, diet, fragment length and microsatellite motif effects on amplification success and genotyping error rates. Conserv Genet 8:249–260
Broquet T, Petit E (2004) Quantifying genotyping errors in noninvasive population genetics. Mol Ecol 13:3601–3608
Bubb A, Ehlers K, Kotze A, Grobler JP (2011) The effect of sample age and storage method on DNA yield and microsatellite amplification from baboon (Papio ursinus) faecal samples. Eur J Wildl Res 57:971–975
Buchan JC, Archie EA, Van Horn RC, Moss CJ, Alberts SC (2005) Locus effects and sources of error in noninvasive genotyping. Mol Ecol 5:680–683
Burnham KP, Anderson DR (2002) Model selection and multimodel inference. A Practical Information-Theoretic Approach. Springer, New York
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35
Carroll EL, Bruford MW, DeWoody JA et al (2018) Genetic and genomic monitoring with minimally invasive sampling methods. Evol Appl 11:1094–1119
Coxon K, Chanin P, Dallas J, Sykes T (1999) The use of DNA fingerprinting to study the population dynamics of otters (Lutra lutra) in Southern Britain: A feasibility study. Research & Development Technial Report W202. Environment Agency, Bristol
Dallas JF, Piertney SB (1998) Primer Notes: Microsatellite primers for the Eurasian otter. Mol Ecol 7:1248–1251
De Barba M, Waits LP (2010) Multiplex pre-amplification for noninvasive genetic sampling: is the extra effort worth it? Mol Ecol Resour 10:659–665
De Barba M, Miquel C, Lobréaux S et al (2017) High-throughput microsatellite genotyping in ecology: improved accuracy, efficiency, standardization and success with low-quantity and degraded DNA. Mol Ecol Resour 17:492–507
Demay SM, Becker PA, Eidson CA et al (2013) Evaluating DNA degradation rates in faecal pellets of the endangered pygmy rabbit. Mol Ecol Resour 13:654–662
Ferreira CM, Sabion-Marques H, Barbosa S et al (2018) Genetic non-invasive sampling (gNIS) as a cost-effective tool for monitoring elusive small mammals. Eur J Wildl Res 64:46
Fike JA, Serfass TL, Beheler AS, Rhodes OE (2004) Genotyping error rates associated with alternative sources of DNA for the North American river otter. IUCN Otter Spec Gr Bull 21:1–16
Frantzen MAJ, Silk JB, Ferguson JWH et al (1998) Empirical evaluation of preservation methods for faecal DNA. Mol Ecol 7:1423–1428
Frosch C, Dutsov A, Zlatanova D, Valchev K, Reiners TE, Steyer K, Pfenninger M, Nowak C (2014) Noninvasive genetic assessment of brown bear population structure in Bulgarian mountain regions. Mamm Biol 79:268–276
Gillet F, Cabria Garrido MT, Blanc F, Fournier-Chambrillon C, Némoz M, Sourp E, Vial-Novella C, Zardoya R, Aulagnier S, Michaux JR (2017) Evidence of fine-scale genetic structure for the endangered Pyrenean desman (Galemys pyrenaicus) in the French Pyrenees. J Mammal 98:523–532
Grueber CE, Nakagawa S, Laws RJ, Jamieson IG (2011) Multimodel inference in ecology and evolution: Challenges and solutions. J Evol Biol 24:699–711
Hájková P, Zemanová B, Bryja J et al (2006) Factors affecting success of PCR amplification of microsatellite loci from otter faeces. Mol Ecol Notes 6:559–562
Hájková P, Zemanová B, Roche K, Hájek B (2009) An evaluation of field and noninvasive genetic methods for estimating Eurasian otter population size. Conserv Genet 10:1667–1681
Hausknecht R, Bayerl H, Gula R, Kuehn R (2010) Application of quantitative real-time polymerase chain reaction for noninvasive genetic monitoring. J Wildl Manag 74:1904–1910
Hurvich CM, Tsai C-L (1989) Regression and time series model selection in small samples. Biometrika 76:297–307
IUCN (2020) The IUCN Red list of threatened species. Version 2020-1. http://www.iucnredlist.org.
Jansman HAH, Chanin PRF, Dallas JF (2001) Monitoring otter populations by DNA typing of spraints. IUCN Otter Spec Gr Bull 18:12–19
Janssens X, Fontaine MC, Michaux JR, Libois R, Kermabon de J, Defourny P, Baret PV (2008) Genetic pattern of the recent recovery of European otters in southern France. Ecography 31:176–186
Klütsch CFC, Thomas PJ (2018) Improved genotyping and sequencing success rates for North American river otter (Lontra canadensis). Eur J Wildl Res 64:16
Kohn MH, Knauer F, Stoffella A, Schröder W, Pääbo S (1995) Conservation genetics of the European brown bear – a study using excremental PCR of nuclear and mitochondrial sequences. Mol Ecol 4:95–103
Kohn MH, Wayne RK (1997) Facts from feces revisited. Trends Ecol Evol 12:223–227
Krawczyk AJ, Bogdziewicz M, Majkowska K, Glazaczow A (2016) Diet composition of the Eurasian otter Lutra lutra in different freshwater habitats of temperate Europe: a review and meta-analysis. Mammal Rev 46:106–113
Kruuk H (2006) Otters: ecology, behaviour and conservation. Oxford University Press, Oxford
Lampa S, Gruber B, Henle K, Hoehn M (2008) An optimisation approach to increase DNA amplification success of otter faeces. Conserv Genet 9:201–210
Lampa S, Henle K, Klenke R et al (2015a) How to overcome genotyping errors in non-invasive genetic mark-recapture population size estimation—a review of available methods illustrated by a case study. J Wildl Manag 77:1490–1511
Lampa S, Mihoub J-B, Gruber B, Klenke R, Henle K (2015b) Non-invasive genetic mark-recapture as a means to study population sizes and marking behaviour of the elusive Eurasian Otter (Lutra lutra). PLoS One 10:e0125684
Lanszki J, Hidas A, Szentes K, Revay T, Lehoczky I, Weiss S (2008) Relative spraint density and genetic structure of otter (Lutra lutra) along the Drava River in Hungary. Mamm Biol 73:40–47
Lerone L, Mengoni C, Carpaneto GM, Randi E, Loy A (2014) Procedures to genotype problematic non-invasive otter (Lutra lutra) samples. Acta Theriol 59:511–520
Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2019) cluster: cluster analysis basics and extensions. R package version 2.1.0. https://cran.r-project.org/web/packages/cluster/.
Martin EA, Heurich M, Müller J, Bufka L, Bubliy O, Fickel J (2017) Genetic variability and size estimates of the Eurasian otter (Lutra lutra) population in the Bohemian Forest Ecosystem. Mamm Biol 86:42–47
Miles KA, Holtz MN, Lounsberry ZT, Sacks BN (2015) A paired comparison of scat-collecting versus scat-swabbing methods for noninvasive recovery of mesocarnivore DNA from an arid environment. Wildl Soc Bull 39:797–803
Monterroso P, Godinho R, Oliveira T, Ferreras P, Kelly MJ, Morin DJ, Waits LP, Alves PC, Mills LS (2019) Feeding ecological knowledge: the underutilised power of faecal DNA approaches for carnivore diet analysis. Mammal Rev 49:97–112
Morin PA, Chambers KE, Boesch C, Vigilant L (2001) Quantitative polymerase chain reaction analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus). Mol Ecol 10:1835–1844
Mowry RA, Gompper ME, Beringer J, Eggert LS (2011) River otter population size estimation using noninvasive latrine surveys. J Wildl Manag 75:1625–1636
Murphy MA, Kendall KC, Robinson A, Waits LP (2007) The impact of time and field conditions on brown bear (Ursus arctos) faecal DNA amplification. Conserv Genet 8:1219–1224
Murphy MA, Waits LP, Kendall KC (2003) The influence of diet on faecal DNA amplification and sex identification in brown bears (Ursus arctos). Mol Ecol 12:2261–2265
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nakamura M, Godinho R, Rio-Maior H, Roque S, Kaliontzopoulou A, Bernardo J, Castro D, Lopes S, Petrucci-Fonseca F, Álvares F (2017) Evaluating the predictive power of field variables for species and individual molecular identification on wolf noninvasive samples. Eur J Wildl Res 63:53
Navidi W, Arnheim N, Waterman MS (1992) A multiple-tubes approach for accurate genotyping of very small DNA samples by using PCR: statistical considerations. Am J Hum Genet 50:347–359
Nsubuga AM, Robbins MM, Roeder AD et al (2004) Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Mol Ecol 13:2089–2094
Panasci M, Ballard WB, Breck S, Rodriguez D, Densmore LD III, Wester DB, Baker RJ (2011) Evaluation of fecal DNA preservation techniques and effects of sample age and diet on genotyping success. J Wildl Manag 75:1616–1624
Piggott MP (2004) Effect of sample age and season of collection on the reliability of microsatellite genotyping of faecal DNA. Wildl Res 31:485–493
Piggott MP, Taylor AC (2003) Extensive evaluation of faecal preservation and DNA extraction methods in Australian native and introduced species. Aust J Zool 51:341–355
Pompanon F, Bonin A, Bellemain E, Taberlet P (2005) Genotyping errors: causes, consequences and solutions. Nat Rev Genet 6:847–859
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/.
Ramón-Laca A, Soriano L, Gleeson D, Godoy JA (2015) A simple and effective method for obtaining mammal DNA from faeces. Wildl Biol 21:195–203
Reed JZ, Tollit DJ, Thompson PM, Amos W (1997) Molecular scatology: the use of molecular genetic analysis to assign species, sex and individual identity to seal faeces. Mol Ecol 6:225–234
Santini A, Lucchini V, Fabbri E, Randi E (2007) Ageing and environmental factors affect PCR success in wolf (Canis lupus) excremental DNA samples. Mol Ecol Notes 7:955–961
Schwartz MK, Luikart G, Waples RS (2007) Genetic monitoring as a promising tool for conservation and management. Trends Ecol Evol 22:25–33
Sefc KM, Payne RB, Sorenson MD (2003) Microsatellite amplification from museum feather samples: effects of fragment size and template concentration on genotyping errors. Auk 120:982–989
Silveira Trinca C, Fernandes Jaeger C, Eizirik E (2013) Molecular ecology of the Neotropical otter (Lontra longicaudis): non-invasive sampling yields insights into local population dynamics. Biol J Linn Soc 109:932–948
Sittenthaler M, Bayerl H, Unfer G, Kuehn R, Parz-Gollner R (2015) Impact of fish stocking on Eurasian otter (Lutra lutra) densities: A case study on two salmonid streams. Mamm Biol 80:106–113
Sittenthaler M, Koskoff L, Pinter K et al (2019) Fish size selection and diet composition of Eurasian otters (Lutra lutra) in salmonid streams: Picky gourmets rather than opportunists? Knowl Manag Aquat Ecosyst 420:29
Sittenthaler M, Kunz F, Szymusik A et al (2018) Fine-scale genetic structure in an eastern Alpine black grouse Tetrao tetrix metapopulation. J Avian Biol 49:e01681
Sittenthaler M, Schöll EM, Leeb C, Haring E, Parz-Gollner R, Hackländer K (2020) Marking behaviour and census of Eurasian otters (Lutra lutra) in riverine habitats: what can scat abundances and non-invasive genetic sampling tell us about otter numbers? Mammal Res 65:191–202
Soto-Calderón ID, Ntie S, Mickala P et al (2009) Effects of storage type and time on DNA amplification success in tropical ungulate faeces. Mol Ecol Resour 9:471–479
Stenglein JL, De Barba M, Ausband DE, Waits LP (2010) Impacts of sampling location within a faeces on DNA quality in two carnivore species. Mol Ecol Resour 10:109–114
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Taberlet P, Griffin S, Goossens B, Questiau S, Manceau V, Escaravage N, Waits LP, Bouvet J (1996) Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Res 24:3189–3194
Taberlet P, Luikart G (1999) Non-invasive genetic sampling and individual identification. Biol J Linn Soc 68:41–55
Taberlet P, Waits LP, Luikart G (1999) Noninvasive genetic sampling: look before you leap. Trends Ecol Evol 14:323–327
Velando A, Morán P, Romero R, Fernández J, Piorno V (2017) Invasion and eradication of the American mink in the Atlantic Islands National Park (NW Spain): a retrospective analysis. Biol Invasions 19:1227–1241
Velli E, Fabbri E, Galaverni M et al (2019) Ethanol versus swabs: what is a better tool to preserve faecal samples for non-invasive genetic analyses? Hystrix 30:24–29
Vergara M, Ruiz-González A, López de Luzuriaga J, Gómez-Moliner BJ (2014) Individual identification and distribution assessment of otters (Lutra lutra) through non-invasive genetic sampling: recovery of an endangered species in the Basque Country (Northern Spain). Mamm Biol 79:259–267
Vynne C, Baker MR, Breuer ZK, Wasser SK (2012) Factors influencing degradation of DNA and hormones in maned wolf scat. Anim Conserv 15:184–194
Waits LP, Paetkau D (2005) Noninvasive genetic sampling tools for wildlife biologists: a review of applications and recommendations for accurate data collection. J Wildl Manag 69:1419–1433
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgements
We are grateful to Kathrin Heissenberger, Lucia Sittenthaler and Michaela Bodner for field assistance. For advise in statistics, we thank Ursula Nopp-Mayr. Sample collection and genetic analyses were carried out within studies funded by the Fisheries Association of Lower Austria and its Regional Fishing Associations (I–V), the Provincial Government of Lower Austria (Amt der Niederösterreichischen Landesregierung—Abteilung Naturschutz) and the European Union (Austrian Programme for Rural Development).
Funding
Open Access funding provided by University of Natural Resources and Life Sciences, Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 192 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sittenthaler, M., Schöll, E.M., Leeb, C. et al. Factors influencing genotyping success and genotyping error rate of Eurasian otter (Lutra lutra) faeces collected in temperate Central Europe. Eur J Wildl Res 67, 2 (2021). https://doi.org/10.1007/s10344-020-01444-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-020-01444-4