Abstract
Attempts to restore Lake Lugano, Switzerland and Italy, from eutrophication have produced weak responses in the target variables (primary productivity and hypolimnetic oxygen concentrations), indicating shortcomings in the underlying eutrophication model. An analysis of monitoring data showed that the decrease in phosphorus concentration, although nearly compliant with restoration targets, produced only slight decreases in primary productivity and no change in hypolimnetic oxygen conditions. These target variables were equally or more sensitive to factors external to trophic state, including plankton structure, which influenced primary productivity, and the depth of mixing during turnovers, which influenced hypolimnetic oxygen. To improve the chance of success, the restoration approach should revise the phosphorus concentration target and explicitly account for the influence of external variation, especially mixing depth.
Similar content being viewed by others
Introduction
In lakes, cultural eutrophication is the most widespread environmental problem (Smith & Schindler, 2009). Eutrophic lakes have undesirable traits, including algal blooms and the release of harmful gases from the sediments. Because these traits reduce the ability of lakes to provide ecosystem services, eutrophic lakes have long been a priority for ecological restoration (Edmondson, 1970). Aquatic scientists attribute eutrophication to excess phosphorus, which comes from sources like municipal waste and fertilisers (Smith et al., 1999). Consequently, measures to restore eutrophic lakes have focussed on reducing phosphorus inputs (Dillon & Rigler, 1975; Jeppesen et al., 2005; Schindler et al., 2016).
A main impetus for the restoration of eutrophic lakes stems from the necessity to mitigate the effects of oxygen depletion on water quality and fish stocks (Vollenweider, 1968). According to lake eutrophication models (Wetzel, 1975; Walker, 1979), oxygen depletion is the end result of a pathway connecting phosphorus, primary productivity and hypolimnetic oxygen consumption (conceptual map in Fig. 1). However, due to lack of data, quantitative assessments of these links have been rare and not always supportive. For example, attempts to model primary productivity from phosphorus have been met with limited success (Smith, 1979; Morin et al., 1999), while relationships between hypolimnetic oxygen and trophic state appear minor or non-existent (Smith, 1979; Müller et al., 2012). Therefore, the hypothesised pathway (Fig. 1) probably overemphasises the links between these variables and falls short of identifying the controlling factors. Further assessments of this pathway are needed, because inadequate assumptions concerning the causal relationships between phosphorus, primary productivity and hypolimnetic oxygenation can hinder the success of restoration efforts.
Conceptual map of the eutrophication model underpinning the restoration of the lake. Adapted from Walker (1979) and Wetzel (1975). OM: organic matter; DO: dissolved oxygen. The model assumes that primary productivity is P limited, that the P effect is a function of concentration (Di Toro et al., 1971) and that the depletion of oxygen in the hypolimnion reflects the productivity of the lake
Main challenges to ecosystem management and restoration include lack of understanding about the system (‘structural uncertainty’) and sensitivity to external variation (Allen et al., 2011). Restoration requires predictive models to plan suitable goals and measures (Lamouroux et al., 2015). Therefore, uncertainty may result in poor predictive models and ineffective management. To reduce uncertainty and improve predictive models, restoration would benefit from evaluations of long-term monitoring data. This approach, known as adaptive management, has been advocated for ecosystem management (Allen et al., 2011), but is not widely used in lake restoration. This is unfortunate because not all eutrophic lakes recover following phosphorus reduction (Jeppesen et al., 2005; Søndergaard et al., 2007). In these cases, evaluations of long-term monitoring results may help direct restoration toward more fruitful directions.
We conducted such an evaluation for Lake Lugano, a deep lake that stretches across the border between Switzerland and Italy (Fig. 2). Lake Lugano underwent eutrophication during the twentieth century, reaching eutrophic state by the 1970s (Barbieri & Mosello, 1992). Swiss and Italian administrations then joined efforts to restore the lake and monitor the results. Previous research has shown that measures to control phosphorus inputs have been effective, causing a three-fold reduction (Lepori, 2019a). In-lake phosphorus concentrations have mirrored this reduction, decreasing from high to moderate (e.g. from 72 to 109 μg Ptot l−1 to 27 to 37 μg Ptot l−1 between 1984 and 2017; Lepori, 2019a). However, the response of key indicators of trophic state (especially deep-water oxygenation) to the decline in phosphorus has been weaker than expected (Lepori et al., 2018b). In addition, research on Lake Lugano and neighbouring lakes has shown that temporal variation in factors external to trophic state (e.g. in climatic conditions and trophic structure) has a strong influence on the lake’s ecosystem and can cause undesirable effects similar to those produced by eutrophication (e.g. reduced hypolimnetic oxygenation; Rogora et al., 2018). Therefore, it seems possible that the pathway connecting phosphorus to oxygen depletion was weaker than anticipated and/or that the influence of external factors weakened the lake’s response to the reduction in phosphorus.
In this study, we examined in detail the response of lake phosphorus concentration → primary productivity → hypolimnetic oxygen pathway (Fig. 1) to investigate the effect of phosphorus reduction on hypolimnetic oxygen. We hypothesised that this effect was weak because (1) primary productivity is relatively unaffected by phosphorus at high-to-moderate concentrations (Anneville & Pelletier, 2000; Müller et al., 2019) and (2) the transmission of effects through the phosphorus-hypolimnetic oxygenation pathway is dampened by external variation (i.e. by variables external to trophic state; Rogora et al., 2018). Based on previous research, we focussed on external variation caused by changes in zooplankton structure (Lepori & Roberts, 2017), surface water temperature (Lepori & Roberts, 2015) and depth of mixing during turnovers (Rogora et al., 2018). We used a long-term (1984–2018) series of data (including phosphorus, primary productivity, dissolved oxygen, plankton and other variables) to evaluate the relative influences of phosphorus and the external variables (zooplankton structure, water temperature and mixing) on primary productivity and hypolimnetic oxygenation. Based on the results, we asked if the eutrophication model and the restoration measures need revision, and what lessons can be derived to improve the effectiveness of lake restoration in the future.
Materials and methods
Study site
Lake Lugano is a deep (maximum depth: 288 m) and relatively large (surface: 49 km2) natural lake located at the southern edge of the Alps (Switzerland and Italy; Fig. 2). Because of the mild regional climate (average air temperature > 10°C) the lake has a warm monomictic regime, turning over once a year between late-winter and early spring (February–March). The lake is divided by a causeway into two basins, the North basin and the South basin (Fig. 2, Table 1). The causeway, completed in 1847, leaves a small passage through which water flows from the N. basin to the S. basin at a rate of 0.38 km3 year−1 (Barbieri & Mosello, 1992). The N. basin, because of the deep and narrow (fjord-like) morphometry and the long water renewal time (12 years, Table 1) is nearly meromictic, i.e. it is nearly-always stratified into a mixolimnion (approximately 0–100 m) and a monimolimnion (100–288 m) of different densities (Barbieri & Mosello, 1992). Since the beginning of monitoring in the early 1980s, complete mixing occurred only twice, in 2005 and 2006, following exceptionally cold winters (Holzner et al., 2009). The S. basin, because of shallower morphometry, is essentially holomictic, although it has sometimes skipped complete mixing following milder-than-usual winters (unpublished results). Concentrations of reactive phosphorus and stoichiometric nitrogen: phosphorus (N:P) ratios observed in the lake since 1984 suggest that the lake’s phytoplankton is P limited, although P limitation probably increased from weak to strong due to a decline in P and an increase in N (Lepori, 2019b).
In 2003, the catchment of Lake Lugano had a population of 195’443 residents and 70’616 tourists (CIPAIS, 2003). The lake’s waters provide part of this population with drinking water (for example, the main water supplier, serving a population of 68’127, obtains 20% of the water from the lake), thermal energy and recreational opportunities. Woodlands (67% of the area), urban development (11%) and summering pastures (9%) are the predominant land uses in the catchment (Ferrario, 2009).
In the late 1970s and early 1980s, when the first plans to restore the lake from eutrophication were developed (D. A., 1982), the lake was severely eutrophic. For example, in 1976, phosphorus concentrations during mixing (March) were 107 μg Ptot l−1 (N. basin, average 0–100 m) and 194 μg Ptot l−1 (S. basin, average 0–70 m), primary productivity was 690 g C m−2 year−1 (S. basin) and near-bottom oxygen was scarce or absent (year round in the N. basin, during summer and autumn in the S. basin; EURATOM, 1977). Although the pre-industrial state of the lake was oligotrophy (Niessen et al., 1992), the main restoration goal is to re-establish mesotrophic conditions throughout the lake (Imboden, 1992).
The reduction of in-lake phosphorus concentrations has been pursued through a reduction of phosphorus inputs through improved sewage management and a ban on the use of phosphate detergents (Lepori & Roberts, 2017). An implicit assumption of the restoration proposal was that reducing phosphorus lowers primary productivity, which should reduce mineralisation and oxygen consumption, allowing hypolimnetic dissolved oxygen to increase (Fig. 1). A quantitative phosphorus concentration target of < 30 μg Ptot l−1 (average annual concentration) was set to achieve these targets (Imboden, 1992). Primary productivity was expected to decrease to less than 150 g C m−2 year−1 (annual cumulative value) and oxygen concentration to increase to more than 4 mg l−1 (Imboden, 1992). Phosphorus and oxygen targets were applied to the mixolimnion (0–100 m) for the meromictic N. basin and to the entire water column for the S. basin (Barbieri & Mosello, 1992).
Scientific literature identifies different boundaries between eutrophic and mesotrophic state (phosphorus concentrations of 20–35 μg l−1, primary productivity of 219–365 g C m−2 year−1 and minimum oxygen concentrations > 6 mg l−1; Likens, 1975; Wetzel, 1975; Nürnberg, 1996). Because of the different boundaries defined in the literature, especially with regard to primary productivity, both local and literature-based targets were used in this study as benchmarks to assess trophic state recovery.
During the study period, the Lake Lugano ecosystem was influenced by physical and biological variation that has little or no connection with eutrophication. A major change in the lake was a sudden restructuring of the plankton community that took place between 1988 and 1989. At this juncture, zooplankton biomass increased, herbivore zooplankton composition shifted toward larger-bodied species and phytoplankton biomass decreased (Lepori, 2019b). Because the main pelagic planktivorous fish (Bleak, Alburnus sp.) suffered a die-off in 1988 and subsequently became extinct in the lake, the change is consistent with a trophic cascade (Lepori, 2019b), although other factors [especially the arrival of the non-native calanoid copepod Eudiaptomus gracilis (G.O. Sars, 1863)] may have been involved. A physical change concerned surface water temperature, which increased in the lake in parallel with global temperatures (Lepori & Roberts, 2015). In addition, the lake experienced year-to-year variation in the depth of mixing during the late-winter turnover. This variation is related to winter weather conditions (cold winters tend to cause deeper mixing than warmer winters) and triggers a chain of effects on the replenishment of epilimnetic phosphorus, the re-oxygenation of the hypolimnetic waters, and the succession of phyto- and zooplankton (Lepori et al., 2018a; Rogora et al., 2018). In this paper we considered variation in plankton community structure, water temperature and mixing depth to represent external variation (i.e. external to the phosphorus → primary productivity → hypolimnetic oxygen pathway identified in Fig. 1, not necessarily external to the lake).
Data sources and analyses
Data were obtained from the database of the long-term monitoring programme of Lake Lugano, which was set up in the early 1980s to monitor the lake’s response to restoration. The database is held at the University of Applied Sciences and Arts of Southern Switzerland, which runs the program on the behalf of the Administration of Canton Ticino, Switzerland. The data were collected monthly during 1984–2018 from two stations, one near the deepest point of the N. basin and one near the deepest point of the S. basin (Fig. 2). The stations will be referred to as N. basin and S. basin, respectively.
Sampling and analytical methods are reported in Online Appendix 1. Briefly, concentrations of total phosphorus (p, in mg Ptot m−3) and dissolved oxygen (DO, in mg O2 l−1) were measured from lake water samples collected at several depths using a Niskin bottle. Phosphorus was analysed by colorimetry after persulfate oxidation, while DO was analysed by Winkler titration. Primary productivity was measured using the light-and-dark bottle method and 14C as a carbon tracer (based on Nielsen, 1965). Sampling methods for water temperature, conductivity, chlorophyll, light and zooplankton are summarised in Online Appendix 1.
Concentrations of phosphorus and DO at discrete depths were used to calculate mass contents and volume-weighted concentrations for different lake layers following Bührer (1979). According to this method, the mass content of a substance in a layer is calculated assuming that the substance’s concentration varies linearly with depth and the geometry of a layer can be approximated by a truncated cone.
Phosphorus concentration in the lake (plake) was described as the yearly average (volume-weighted) concentration across the 0–100 m mixolimnion (N. basin) or the whole water column (S basin). Although in principle PPR should depend on the concentration within the euphotic zone (peu), exploratory analyses showed that plake and peu were strongly correlated (r = 0.9) and could be used nearly interchangeably as predictors of PPR (results not shown). In this paper plake was used because it is used in the scientific literature to determine trophic state and facilitates comparison to water quality standards (e.g. Nürnberg, 1996).
Primary productivity was described by the total (cumulative) yearly productivity of the lake. Yearly primary productivity was calculated from measured hourly photosynthetic rates and other variables (chlorophyll, temperature and light) through a complex series of interpolations, described in detail in Cannata et al. (2020).
Hypolimnetic DO conditions were described using two variables, the areal hypolimnetic oxygen deficit (AHOD) and the duration of hypoxic conditions at the bottom of the mixolimnion (N. basin) or the bottom of the lake (S. basin; annual duration of hypoxia, DOH). The AHOD was calculated as the rate of change in dissolved oxygen (mg day−1) per unit area of the hypolimnion surface (cm2) during the stratification period (Strøm, 1931 in Wetzel, 1975). For each month, we calculated the volume-weighted DO hypolimnetic concentration as described above (Bührer, 1979). For the N. basin, the hypolimnion was defined as the 20–100 m layer, which corresponds to the layer of the mixolimnion that is seasonally isolated from surface waters (in contrast with the monimolimnion, which never mixes; Gulati et al., 2017). For the S. basin, the hypolimnion was defined as the 20–95 m layer. Based on these monthly concentrations, for each year, we identified the date of maximum hypolimnetic DO concentration during January-May, which marks the turnover, and the first date of departure from a linear decline in DO concentration during September-December. This period, excluding the last date, corresponds to the stratification period, during which hypolimnetic oxygen in Lake Lugano declines at an approximately constant rate due to consumption and lack of replenishment. Monthly hypolimnetic DO concentrations within the stratification period were transformed into areal content by multiplying by the hypolimnion’s volume and dividing by the hypolimnion’s upper surface (Wetzel & Likens, 2000). The AHOD was calculated from these data as the slope of the regression between time (day) and areal hypolimnetic DO content (mg cm−2; Wetzel & Likens, 2000; Rogora et al., 2018).
The index DOH is the number of days per year during which DO concentrations near the bottom of the hypolimnion are in the hypoxic range (North et al., 2014). This index was calculated by interpolating DO concentrations at sampling depths of 75 m (N. basin) and 93 m (S. basin) to 1-day resolution using a cubic spline and counting the days during which DO concentrations were continuously below 4 mg l−1 (restoration target).
An index of surface water temperature (SWT, in °C) relevant to PPR was calculated as the average yearly temperature across the 0–20 m layer, which includes the euphotic zone. The depth of mixing during late-winter turnovers (MIXDEPTH) was estimated as the maximum depth at which conductivity (adjusted to 20°C) differed by > 3 μS cm−1 from the surface values (0–2 m) during the late-winter turnover (Lepori et al., 2018b). The community structure of herbivorous zooplankton (PLANKSTRUCTURE) was described using a dummy variable (0 = 1984–1989, 1 = 1989–2018; rationale in Lepori, 2019b).
The full pathway expected to connect phosphorus concentration to hypolimnetic oxygen content, including AHOD, is plake → PPR → AHOD → DOH. The assessment of the pathway included an evaluation of pairwise effects at individual links and an evaluation of the strength of the whole pathway. Relationships at each link were assessed using linear or log-linear regression models. In addition, the p – PPR relationship was assessed using a linearized version of a hyperbolic function (1/PPR vs. 1/p; Megard et al., 1979), which mimics a saturating effect. For each regression analysis, assumptions of no serial correlation and normal distribution of the residuals were tested using Durbin-Watson (Durbin & Watson, 1950) and Anderson–Darling (Anderson & Darling, 1952) tests. The significance of the predictors in the models was tested using t-tests, whereas the predictive ability was assessed using R2 (values of R2 > 0.5 were considered good; Moriasi et al., 2007). In addition, the effect size of each pairwise relationship was quantified using standardised regression coefficients (path coefficients β; Wright, 1934) and the strength of the whole pathway was assessed using path analysis rules (Wright, 1934). Values of β < 0.1 were considered to indicate weak effects, values near 0.3 a moderate effect and values > 0.5 a strong effect (Cohen, 1988).
The influence of external variability was addressed by evaluating the effects PLANKSTRUCTURE (pre- and post-1989), SWT and MIXDEPTH on the plake → DOH pathway. PLANKSTRUCTURE was expected to influence PPR because food chains with less (and/or smaller-bodied) herbivorous zooplankton are predicted to exert less control on phytoplankton biomass and productivity than food chains with more (and/or larger-bodied) herbivorous zooplankton (Carpenter et al., 2010). Water temperature (SWT) was expected to influence PPR because phytoplankton growth tends to increase exponentially with water temperature (e.g. Eppley, 1972). MIXDEPTH was expected to influence AHOD and DOH because deeper mixing increases the hypolimnetic replenishment of DO before the onset of stratification, and that greater replenishments can delay DO depletion (Schwefel et al., 2016; Rogora et al., 2018). The effects were assessed by adding PLANKSTRUCTURE, SWT and MIXDEPTH as predictors to regression models and estimating the path coefficients of the links connecting these external variables to the main pathway. A standardised path coefficient was not estimated for the effect of the dummy variable PLANKSTRUCTURE on PPR because dummy variables cannot be standardised meaningfully. In this case we resorted to assessing the effect of plake (predictor) on PPR (response) after adjusting for the effect of PLANKSTRUCTURE (categorical variable) using ANCOVA (Werts & Linn, 1969). The relative effects of plake and PLANKSTRUCTURE on PPR were examined based on unstandardised regression coefficients.
The analysis of the plake → PPR → AHOD → DOH pathway was preceded by an analysis of the temporal patterns of each variable. Trends were tested using the Mann–Kendall (MK) test unless serial correlation was detected (i.e. if the lag-one autocorrelation coefficient was significantly different from zero), in which case a modified test known as the ‘Trend-free pre-whitening MK test’ (TFPW-MK; Yue et al., 2002) was used. To assess if mesotrophic conditions were achieved by 2018, the trends were plotted against literature-based values indicative of the eutrophic-mesotrophic transition (plake: 35 μg l−1, PPR: 365 g C m−2 year−1; AHOD:0.04 mg day−1 cm−2 and DOH = 0 days yr−1; Wetzel, 1975; Nürnberg, 1996).
Results
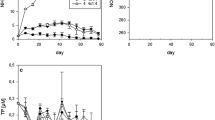
The analysis of temporal patterns (Table 2, Fig. 3) indicated that, between 1984 and 2018, plake decreased in both basins, PPR decreased (slightly) in the S. basin, whereas all other variables displayed no trends. In the N. basin, the trend of plake was interrupted between the late 1990s and (approximately) 2010 by a transitory increase, which peaked during 2005–2006, the years of the exceptional turnovers. Despite this interruption, by the end of the study period, plake decreased by 61–86% (Table 2), reaching values considered typical of mesotrophic state (< 35 μg l−1) in both basins. By comparison, PPR decreased only in the S. basin (− 36%) and hypolimnetic DO conditions (indicated by AHOD and DOH) did not change. Throughout the study period, both lake basins experienced annual periods of hypolimnetic hypoxia lasting nearly-always more than 200 days.
Linear regressions of 1/PPR vs. 1/plake displayed poorer fit than regressions of PPR vs. log10(plake) (results not shown), indicating that the pattern between PPR and plake was better described by a log-linear rather than a hyperbolic function. Based on log-linear models, plake had significant positive effects on PPR (Table 3, Fig. 4), although the predictive accuracy was weak to moderate (R2 = 20.1% for the N. basin, R2 = 45.6% for the S. basin). After adding the external predictors zooplankton structure (PLANKSTRUCTURE) and water temperature (SWT) to the model, the effect of plake remained significant in both basins, PLANKSTRUCTURE had an effect in the S. basin and SWT had no effects. The effect of PLANKSTRUCTURE was negative, indicating that the pre-1989 structure was associated with higher PPR for the same concentration of plake than the post-1989 structure (an additional 132 g C m−2 year−1 on average). The partial regression coefficient of log10(plake) decreased from 345 to 193, indicating that part of the effect attributed to plake in the plake-PPR model was explained by the change in plankton structure. Predictive abilities increased to moderate (N. basin: R2 = 29.7%) and good (S. basin: R2 = 58.0%). The path coefficients of the plake-PPR relationship indicate moderate-to-strong effects (N. basin: β = 0.49; S. basin: β = 0.51 after removing the effect of PLANKSTRUCTURE).
Relationships between plake, PPR, AHOD and DOH in the two main basins of Lake Lugano during 1984–2018. See Table 3. The two segments in the PPR versus plake relationship represent differences in PLANKSTRUCTURE (small-bodied zooplankton before 1989 in dark green, large-bodied zooplankton after 1989 in lighter green)
The areal hypolimnetic oxygen deficit AHOD was unrelated to PPR in both basins (Table 3, Fig. 4). Adding log10(MIXDEPTH) as a predictor to the regression model resulted in a non-significant model for the N. basin and a significant model for the S. basin, in which both PPR and log(MIXDEPTH) had significant effects. For the S. basin, the predictive accuracy R2 increased from 8.2% to 28.3%, although it remained moderate. Path coefficients (Fig. 5) indicate that in the S. basin the effect of PPR on AHOD was strong (β = 0.55) and the effect of log(MIXDEPTH) was moderate to strong (β = 0.48). In this basin, the effect of plake on AHOD through PPR was moderate (0.51 × 0.55 = 0.28).
Path coefficients linking plake, PPR, AHOD and DOH in the study lake. Adj. PPR is PPR adjusted for the effects of PLANKSTRUCTURE using ANCOVA. The thickness of the arrows indicates the strength of the effects (see Methods). †Nearly significant (P = 0.058), other symbols as in Table 1
The duration of hypoxia (DOH) at the bottom of the hypolimnion was unrelated to AHOD in both lakes basins (Table 3, Fig. 4). Adding log(MIXDEPTH) as a predictor to the regression model resulted in significant models in both basins. The effect was negative, meaning that deeper mixing resulted in shorter DOH. However, in the N. basin, the effect of log(MIXDEPTH) was significant only if the two years of the exceptional turnovers (2005–2006), which were outliers in the MIXDEPTH–DOH regression, were excluded. In these years, waters at 75 m of depth remained continuously hypoxic, indicating that very deep mixing (> 100 m) had negative effects on hypolimnetic oxygenation. The direct effects of log(MIXDEPTH) on DOH were strong (β = − 0.61 and − 0.57, Fig. 5). The indirect effects (via AHOD) were weak to negligible (N. basin: β = + 0.01, S. basin: β = − 0.11). The overall effects of plake on DOH through the plake → PPR → AHOD → DOH pathway were negligible (N. basin: β =+0.01, S. basin: β = − 0.06).
Discussion
In Lake Lugano, thanks to decades of phosphorus control (Lepori & Roberts, 2017), phosphorus concentrations (plake) decreased throughout the study period. One notable exception to this trend was the transient increase in plake observed in the N. basin between the late 1990s and 2005–2006. This increase was caused by an upwell of phosphorus from the monimolimnion into the mixolimnion linked to a process of chemocline erosion. This process included an initial weakening of the stratification during 1995–2005, caused by a period of warming of the hypolimnion (Holzner et al., 2009), and the exceptional mixing events of 2005–2006, which were triggered by colder-than-usual winters south of the Alps (Salmaso et al., 2014). Despite this exception, toward the end of the study period, phosphorus concentrations met (N. basin) or nearly met (S. basin) the local restoration objective of 30 μg l−1. Moreover, according to literature-based thresholds (35 μg l−1; Nürnberg, 1996) both basins reached phosphorus concentrations typical of mesotrophic lakes. In comparison, primary productivity (PPR) and hypolimnetic DO conditions, the main targets of the restoration projects, changed little or not at all, remaining within ranges typical of eutrophic waters. Therefore, the reduction of phosphorus concentrations was not followed by the desired ecosystem changes (PPR below 150 g C m−2 year−1 and DO above 4 mg l−1).
Supporting our hypotheses, the link between p and hypolimnetic DO conditions was weak for two reasons. First, within the high-to-moderate range of p observed in the lake, PPR changed relatively little with p. Second, because of the influence by external factors (zooplankton structure and depth of mixing), even those effects of p reduction that were detected on PPR were not transmitted to hypolimnetic oxygen conditions. These results indicate that the restoration was based on simplistic assumption concerning the lake’s ecosystem, which indicates structural uncertainty. Because the restoration is ongoing, improving knowledge about the p → DOH pathway could help revise the restoration’s assumptions and enhance the project’s potential to meet its goals.
Controls of primary productivity
Although phosphorus influenced primary productivity, the overall effect was modest. For example, the regression models developed in this study predicted that a reduction of plake from 100 to 30 μg l−1 (i.e. from values typical of the early 1980s to the restoration target) would reduce PPR from 529–570 to 423–464 g C m−2 year−1 (N. basin-S. basin). In other words, a 70% reduction in plake would result only in 17–19% reductions in PPR. Unfortunately, the values of plake needed to reduce PPR to the restoration targets (local target: 150 g C m−2 year−1, literature-based target: 365 g C m−2 year−1) cannot be predicted from the models because these targets lie well beyond the observed data.
Patterns of PPR in lakes have been described as a saturating function of phosphorus concentration (Smith, 1979). However, in contrast with a saturation model, in Lake Lugano, the relationship between PPR and plake was apparent even at high phosphorus concentrations (up to concentrations of approximately 100 µg l−1) and the relationship was better described by a log-linear rather than a hyperbolic function. This difference aside, either log-linear or hyperbolic (saturating) patterns indicate that the effect of phosphorus on PPR declines with increasing phosphorus concentrations. From a restoration practice viewpoint, a log-linear or hyperbolic relationship means that phosphorus reductions have weak effects on PPR above moderate phosphorus concentrations, but may become effective at lower phosphorus concentrations. Based on (scarce) empirical evidence, the threshold between ‘moderate’ and ‘low’ phosphorus concentrations lies around 20 µg l−1(Smith, 1979), a threshold which is well below the plake target for Lake Lugano’s restoration.
Weak or no effects of moderate phosphorus reductions on primary productivity are not uncommon in deep lakes (e.g. Anneville & Pelletier, 2000; Gammeter & Zimmermann, 2000) and have been attributed to changes in phosphorus uptake and underwater light conditions (Fitzpatrick et al., 2007). First, phytoplankton can compensate for potential phosphorus limitation by taking up inorganic phosphate more efficiently (Wagner & Falkner, 2001) or by taking up soluble organic phosphate thanks to extracellular phosphatase enzymes (Whitton et al., 2005). Second, increased light penetration reduces the self-shading effect of phytoplankton and allows photosynthesis to occur in deeper layers, compensating for the reduced phosphorus availability (Sas, 1990). Additionally, some species of phytoplankton are efficient at enhancing photosynthetic potential under low light conditions in deeper waters and can sustain high levels of productivity in mesotrophic environments (Reynolds, 2006). Therefore, the range between eutrophic and mesotrophic states may span a domain of ‘diminishing returns’, where reductions in phosphorus cause proportionately small reductions in primary productivity. This effect has led researchers to believe that, at least in deep Alpine lakes, highly P-enriched (eutrophic) and moderately P-enriched (mesotrophic) lakes do not differ substantially in terms of productivity or phytoplankton biomass (Anneville & Pelletier, 2000; Gammeter & Zimmermann, 2000).
PPR was also controlled by zooplankton structure (species and size composition), although the effect emerged only in the S. basin. As expected, the post-1989 community, characterised by greater biomass of herbivorous zooplankton and dominance by large-bodied species, was associated with lower primary productivity per unit phosphorus, suggesting a suppression of phytoplankton through consumption. If the change in structure was prompted by the decline of planktivory (see Methods), the suppression of phytoplankton is consistent with an archetypical trophic cascade (Carpenter et al., 2010). Moreover, because phosphorus concentrations were generally higher in the S. basin, the stronger effect of zooplankton structure in the S. basin is consistent with a trophic cascade hypothesis called “Increasing difference” (Carpenter et al., 2010), which predicts that the scope for trophic cascades is greater at higher nutrient inputs. A surprising consequence of this effect is that most of the decline in PPR observed in the S. basin should be attributed to the change in zooplankton structure, not to the decline in phosphorus. In other words, failing to account for the change in community structure would lead to overestimating the effect of the phosphorus decline on PPR. This result highlights the role of zooplankton in lake food webs and illustrates how zooplankton-monitoring data can be a prerequisite to understand the causes of ecological change in lakes.
Although the growth of phytoplankton is temperature dependent, in this study, we did not detect any effects of water temperature in the euphotic zone (0–20 m) on PPR. According to our results (not shown), the temperature of the euphotic zone (0–20 m) of both lake’s basins increased by 1.6°C on average during 1984–2018. Based on Eppley’s curve (Eppley, 1972), and given initial yearly average temperatures of 10.5°C and 9.9°C (N.basin and S. basin, respectively), this temperature change would increase the maximum attainable growth of phytoplankton by 1%. Therefore, any difference in potential growth caused by changes in temperature was probably too small to be detected from our field observations of primary productivity.
Controls of hypolimnetic DO
The expected effect of PPR on hypolimnetic DO consumption (AHOD) emerged only in the S. basin. The reasons for the difference between basins are not clear but might relate to the water layers considered (the true hypolimnion in the S. basin, which is in contact with the sediments, the lower part of the mixolimnion in the N. basin, which lies above the monimolimnion). The main processes that cause AHOD in lakes are (1) the mineralisation and respiration of PPR settling in the water column, (2) the further processing of PPR recently deposited at the sediment’s surface and (3) the oxidation of reduced substances (e.g. ammonium, hydrogen sulphide and methane) released from the sediments (Müller et al., 2019). A strong coupling between PPR and AHOD should arise where/when processes (1) and (2) are predominant, whereas process (3) could weaken the PPR-AHOD link. Therefore, the lack of coupling between PPR and AHOD observed in the 20–100 m layer of the N. basin could have two causes. First, in the N. basin process (3) could be more important, perhaps because the monimolimnion provides a larger and more constant supply of reduced substances (the sediments of the N. basin are always anoxic and should release reduced substances year round, whereas the sediments of the S. basin become anoxic only during the stratification period). Second, in the N. basin process (2) could be less important, because the 20–100 m layer does not include the sediments. To unravel these hypotheses, the relative importance of the oxygen-consuming processes in the two basins of the lake should be researched further.
In the S. basin, AHOD was further positively related to the depth of mixing (MIXDEPTH), meaning that deeper turnovers were followed by faster rates of hypolimnetic DO consumption. In this basin, deep waters became anoxic during the stratification period and were re-oxygenated only during late-winter turnovers if mixing extended all the way to the bottom (unpublished results). Shallower mixing caused non-stop anoxia until the following year. The positive effect of MIXDEPTH on AHOD suggests that oxygen-consuming processes were enhanced by the oxic conditions that followed full turnovers, and hindered by the anoxic conditions that followed shallower turnovers. Similarly, Rogora et al. (2018) observed that in deep southern Alpine lakes rates of DO consumption during summer stratification were higher following complete turnovers. Because deeper turnovers in these lakes are accompanied by greater hypolimnetic DO replenishment, the effect is consistent with the idea that mineralisation and respiration of organic matter in the hypolimnion is a function of ambient DO, at least at low DO concentrations (Burns, 1995).
The duration of hypoxia DOH was not explained by AHOD, indicating a severed link in the expected phosphorus → hypolimnetic DO pathway. Instead, in both basins DOH was influenced by the depth of mixing during the late-winter turnover. In deep lakes, deeper mixing allows a build-up of a greater hypolimnetic oxygen reserve before the onset of the stratification period. Therefore, the positive effect of MIXDEPTH on DOH indicates that the duration of hypoxia depended mainly on the oxygen reserve available at the beginning of the stratification period. A large reserve delayed or prevented the development of hypoxia, whereas a small reserve (no reserve in years when mixing did not reach the deeper waters) lead to early or constant hypoxia. A similar effect was observed in Lake Geneva, where, based on numerical modelling, deep-water hypoxia was influenced more strongly by mixing in winter than by long-term variation in trophic status (Schwefel et al., 2016).
In the S. basin, the depth of mixing had opposing effects on hypolimnetic DO conditions, because it favoured faster depletion (AHOD) and reduced the duration of hypoxia (DOH) at the same time. However, the rate of depletion AHOD had no effects on DOH, indicating that the net effect of the depth of mixing on deep-water oxygen concentrations was positive.
The net effect of MIXDEPTH on DOH indicates that warming climate, like eutrophication, can have negative effects on hypolimnetic oxygenation. In deep temperate lakes, MIXDEPTH is reduced during warmer winters (Salmaso et al., 2014; Rogora et al., 2018). Therefore, a trend toward warmer winters will result in reduced mixing and, all else remaining equal, longer DOH. Lake Lugano, like other lakes worldwide, is on a warming trend that is projected to continue at least until the end of the 21st century (Lepori & Roberts, 2015). The warming trend will work against the restoration of hypolimnetic oxygenation, suggesting that current restoration goals may not be met without an intensification of restoration efforts.
An exception to the positive effect of mixing on DOH was observed in the N. basin during the exceptional turnovers (2005–2006). In these years, our results show that mixing resulted in constant hypoxic conditions at 75 m of depth because it caused an upward flux of reduced substances (methane, ammonium and hydrogen sulphide), which consumed, rather than replenished, the oxygen content across the mixolimnion (Holzner et al., 2009). Therefore, in meromictic lakes with anoxic monimolimnia, deeper mixing lead to greater hypolimnetic oxygenation only when the mixing depth does not exceed the depth of the chemocline.
The inconsistent relationship between plake and AHOD and DOH observed in this study and the lack of AHOD and DOH recovery during the study period agree with a recent study of Swiss lakes, which showed that hypolimnetic oxygen consumption remains consistently elevated and nearly-constant at moderate-to-high levels of phosphorus inputs (> 0.54 g P m−2; Müller et al., 2019). Therefore, like for primary productivity, moderate-to-high phosphorus inputs (or in-lake concentrations, which broadly reflect inputs) represent a domain of little or no change for hypolimnetic oxygen consumption. In contrast, the hypolimnetic oxygen consumption declines steeply with declining P for P inputs below 0.54 g P m−2. These results suggest that phosphorus reductions can have beneficial effects on hypolimnetic oxygen conditions, presumably through reductions in PPR, but only at low P inputs. In 2013–2017, in Lake Lugano, average areal P loadings were 0.6 g P m−2 (N. basin) and 1.4 g P m−2 (S. basin; Lepori, 2019b), therefore still in the domain of no effect.
Concluding remarks
The weak association observed between phosphorus and hypolimnetic oxygenation does not mean that phosphorus control is an ineffective restoration measure. On the contrary, phosphorus reduction can be effective (Jeppesen et al., 2005) and is well suited to large eutrophic lakes, where alternative restoration options (e.g. hypolimnetic aeration, sediment phosphorus locking) are unpractical. However, our results question the restoration assumption that reducing phosphorus from high-to-moderate concentrations is sufficient to achieve the desired changes in primary productivity and hypolimnetic oxygen conditions. Because the restoration of Lake Lugano is ongoing, restoration assumptions and targets could be adapted in the future to improve the possibility of success.
Based on our results, we suggest that meeting restoration goals will require a substantial revision of the phosphorus concentration target to account for the non-linear (‘diminishing-return’) response of PPR and the sensitivity of the p → DOH pathway to external variability, including climatic variation. Current best practices for ecosystem management and ecological forecasting suggest that the following actions could increase the effectiveness of restoration: (1) develop and/or calibrate an ecosystem model for Lake Lugano, e.g. using coupled hydrodynamic and ecosystem process-based models (Vinçon-Leite & Casenave, 2019); (2) forecast the lake ecosystem effects of climate change based on these models and climate scenarios; (3) derive phosphorus concentration targets to avoid hypoxic conditions (DO < 4 mg l−1) under present and future climate scenarios; (4) develop and implement restoration actions needed to achieve the new phosphorus concentration targets; (5) refine monitoring designs to ensure that all the relevant information needed to develop/calibrate the ecosystem model are measured and (6) re-assess regularly models and climate scenarios against the lake’s response, based on monitoring data, following an adaptive management cycle. Model outputs are inherently uncertain, especially with regard to biological responses (Vinçon-Leite & Casenave, 2019). Therefore, an adaptive approach would seem especially useful to constrain uncertainty, generate robust models and increase management effectiveness.
References
Allen, C. R., J. J. Fontaine, K. L. Pope & A. S. Garmestani, 2011. Adaptive management for a turbulent future. Journal of Environmental Management 92: 1339–1345.
Anderson, T. W. & D. A. Darling, 1952. Asymptotic theory of certain `goodness-of-fit’ criteria based on stochastic processes. Annals of Mathematical Statistics 23: 193–212.
Anneville, O. & J. P. Pelletier, 2000. Recovery of Lake Geneva from eutrophication: quantitative response of phytoplankton. Archiv für Hydrobiologie 148: 607–624.
Barbieri, A. & R. Mosello, 1992. Chemistry and trophic evolution of Lake Lugano in relation to nutrient budget. Aquatic Sciences 54: 219–237.
Bührer, V. H., 1979. Die Berechnung der totalen Menge gelöster Stoffe in Seen. Schweizerische Zeitschrift für Hydrologie 41(2): 418–420.
Burns, N. M., 1995. Using hypolimnetic dissolved oxygen depletion rates for monitoring lakes. New Zealand Journal of Marine and Freshwater Research 29: 1–11.
Cannata, M., Lepori, F. & Capelli C. (2020). SoftLake (Version beta-0.1). Zenodo. http://doi.org/10.5281/zenodo.3618219
Carpenter, S. R., Cole, J. J., Kitchell, J. F. & Pace, M. L. (2010). Trophic cascades in lakes: lessons and prospects. In Terborgh, J. & Estes, J.A. (eds), Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature. Island Press: 55-69.
CIPAIS, 2003. Rapporto sullo stato attuale e fabbisogni di opere per la protezione delle acque italo-svizzere. Italy, Turin.
Cohen, J., 1988. Statistical power analysis for the behavioural sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates.
D.A., Dipartimento Ambiente [del Cantone Ticino], 1982. Il Lago Ceresio. Stato delle sue acque, obiettivi, misure d’intervento. [Lake Lugano: State of its waters, restoration schemes and goals]. Bellinzona, 85 pp.
Di Toro, D. M., D. J. O’Connor & R. V. Thomann, 1971. A dynamic model of the phytoplankton population in the Sacramento—San Joaquin Delta. Advances in Chemistry 106: 131–180.
Dillon, P. J. & F. H. Rigler, 1975. A simple method for predicting the capacity of a lake for development based on lake trophic status. Journal of the Fisheries Board of Canada 32: 519–1531.
Durbin, J. & G. S. Watson, 1950. Testing for Serial Correlation in Least Squares Regression, I. Biometrika 37: 409–428.
Edmondson, W. T., 1970. Phosphorus, nitrogen, and algae in Lake Washington after diversion of sewage. Science 169: 690–691.
Eppley, R. W., 1972. Temperature and phytoplankton growth in the sea. Fishery Bulletin 70: 1063–1085.
EURATOM (1977) Studio sull’eutrofizzazione del Lago di Lugano. Campagna 1976. Commissione delle Comunità Europee, CCR EURATOM, Ispra, Italy
Ferrario, L. (2009) Quantificazione e caratterizzazione dei carichi di nutrienti in entrata al Lago di Lugano (Svizzera-Italia). Master’s thesis. Università degli Studi dell’Insubria, Varese, Italy.
Fitzpatrick, M. A., M. Munawar, J. H. Leach & G. D. Haffner, 2007. Factors regulating primary production and phytoplankton dynamics in western Lake Erie. Fundamental and Applied Limnology/Archiv für Hydrobiologie 169: 137–152.
Gammeter, S. & U. Zimmermann, 2000. Changes in phytoplankton productivity and composition during reoligotrophication in two Swiss lakes. SIL Proceedings 1922–2010(27): 2190–2193.
Gulati, R. D., E. S. Zadereev & A. G. Degermendzhi (eds), 2017. Ecology of Meromictic Lakes. Springer, Cham.
Holzner, C. P., W. Aeschbach-Hertig, M. Simona, M. Veronesi, D. M. Imboden & R. Kipfer, 2009. Exceptional mixing events in meromictic Lake Lugano (Switzerland/Italy), studied using environmental tracers. Limnology and Oceanography 54: 1113–1124.
Imboden, D. M., 1992. Possibilities and limitations of lake restoration: conclusions for Lake Lugano. Aquatic Sciences 54: 381–390.
Jeppesen, E., M. Søndergaard, J. P. Jensen, K. E. Havens, O. Anneville, L. Carvalho, et al., 2005. Lake responses to reduced nutrient loading–an analysis of contemporary long-term data from 35 case studies. Freshwater Biology 50: 1747–1771.
Lamouroux, N., J. A. Gore, F. Lepori & B. Statzner, 2015. The ecological restoration of large rivers needs science-based, predictive tools meeting public expectations: an overview of the Rhône project. Freshwater Biology 60: 1069–1084.
Lepori, F., 2019a. Il risanamento del Lago di Lugano: tendenze pluridecennali dei carichi e delle concentrazioni di fosforo. Bollettino della Società ticinese di Scienze naturali 107: 13–19.
Lepori, F., 2019b. Effects of zooplankton structure and phosphorus concentration on phytoplankton biomass in a freshwater pelagic food chain. Fundamental and Applied Limnology/Archiv für Hydrobiologie 192: 305–317.
Lepori, F. & J. J. Roberts, 2015. Past and future warming of a deep European lake (Lake Lugano): what are the climatic drivers? Journal of Great Lakes Research 41: 973–981.
Lepori, F. & J. J. Roberts, 2017. Effects of internal phosphorus loadings and food-web structure on the recovery of a deep lake from eutrophication. Journal of Great Lakes Research 43: 255–264.
Lepori, F., J. J. Roberts & T. S. Schmidt, 2018a. A paradox of warming in a deep peri-Alpine lake (Lake Lugano, Switzerland and Italy). Hydrobiologia 824: 215–228.
Lepori, F., M. Bartosiewicz, M. Simona & M. Veronesi, 2018b. Effects of winter weather and mixing regime on the restoration of a deep perialpine lake (Lake Lugano, Switzerland and Italy). Hydrobiologia 824: 229–242.
Likens, G. E., 1975. Primary production of inland aquatic ecosystems. In Lieth, H. & R. H. Whittaker (eds), Primary productivity of the biosphere. Springer, Berlin: 185–202.
Megard, R. O., W. S. Combs Jr., P. D. Smith & A. S. Knoll, 1979. Attenuation of light and daily integral rates of photosynthesis attained by planktonic algae 1. Limnology and Oceanography 24: 1038–1050.
Moriasi, D. N., J. G. Arnold, M. W. Van Liew, R. L. Bingner, R. D. Harmel & T. L. Veith, 2007. Model evaluation guidelines for systematic quantification of accuracy in watershed simulations. Transactions of the ASABE 50: 885–900.
Morin, A., W. Lamoureux & J. Busnarda, 1999. Empirical models predicting primary productivity from chlorophyll a and water temperature for stream periphyton and lake and ocean phytoplankton. Journal of the North American Benthological Society 18: 299–307.
Müller, B., L. D. Bryant, A. Matzinger & A. Wüest, 2012. Hypolimnetic oxygen depletion in eutrophic lakes. Environmental Science and Technology 46: 9964–9971.
Müller, B., T. Steinsberger, R. Schwefel, R. Gächter, M. Sturm & A. Wüest, 2019. Oxygen consumption in seasonally stratified lakes decreases only below a marginal phosphorus threshold. Scientific reports 9: 1–7.
Nielsen, E. S., 1965. On the determination of the activity in 14C-ampoules for measuring primary production. Limnology and Oceanography 10(suppl): 247–252.
Niessen, F., L. Wick, G. Bonani, C. Chondrogianni, and C. Siegenthaler, 1992. Aquatic system response to climatic and human changes: Productivity, bottom water oxygen status, and sapropel formation in Lake Lugano over the last 10 000 years. Aquatic Sciences 54: 257–276.
North, R. P., R. L. North, D. M. Livingstone, O. Köster & R. Kipfer, 2014. Long-term changes in hypoxia and soluble reactive phosphorus in the hypolimnion of a large temperate lake: consequences of a climate regime shift. Global Change Biology 20: 811–823.
Nürnberg, G. K., 1996. Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake and Reservoir Management 12: 432–447.
Reynolds, C. S. (2006) The Ecology of Phytoplankton. Cambridge University Press, Cambridge.
Rogora, M., F. Buzzi, C. Dresti, B. Leoni, F. Lepori, R. Mosello, M. Patelli & N. Salmaso, 2018. Climatic effects on vertical mixing and deep-water oxygen content in the subalpine lakes in Italy. Hydrobiologia 824: 33–50.
Salmaso, N., F. Buzzi, L. Cerasino, L. Garibaldi, B. Leoni, G. Morabito, M. Rogora & M. Simona, 2014. Influence of atmospheric modes of variability on the limnological characteristics of large lakes south of the Alps: a new emerging paradigm. Hydrobiologia 731: 31–48.
Sas, H., 1990. Lake restoration by reduction of nutrient loading: expectations, experiences, extrapolations. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 24: 247–251.
Schindler, D. W., S. R. Carpenter, S. C. Chapra, R. E. Hecky & D. M. Orihel, 2016. Reducing phosphorus to curb lake eutrophication is a success. Environmental Science & Technology 50: 8923–8929.
Schwefel, R., A. Gaudard, A. Wüest & D. Bouffard, 2016. Effects of climate change on deepwater oxygen and winter mixing in a deep lake (Lake Geneva): comparing observational findings and modeling. Water Resources Research 52: 8811–8826.
Smith, V. H., 1979. Nutrient dependence of primary productivity in lakes 1. Limnology and Oceanography 24: 1051–1064.
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology and Evolution 24: 201–207.
Smith, V. H., G. D. Tilman & J. C. Nekola, 1999. Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environmental Pollution 100: 179–196.
Søndergaard, M., E. Jeppesen, T. L. Lauridsen, C. Skov, E. H. van Nes, R. M. M. Roijackers, E. Lammens & R. Portielje, 2007. Lake restoration: successes, failures and long-term effects. Journal of Applied Ecology 44: 1095–1105.
Vinçon-Leite, B. & C. Casenave, 2019. Modelling eutrophication in lake ecosystems: a review. Science of the Total Environment 651: 2985–3001.
Vollenweider, R. A. (1968) The Scientific Basis of Lake Eutrophication, with Particular Reference to Phosphorus and Nitrogen as Eutrophication Factors; Technical Report DAS/DSI/68.27; Organisation for Economic Co-operation and Development (OECD): Paris, 1968; p 159.
Wagner, F. & G. Falkner, 2001. Phosphate limitation. In Rai, L. C. & J. P. Gaur (eds), Algal Adaptation to Environmental Stresses. Springer, Heidelberg: 65–110.
Walker Jr., W. W., 1979. Use of hypolimnetic oxygen depletion rate as a trophic state index for lakes. Water Resources Research 15: 1463–1470.
Werts, C. E. & R. L. Linn, 1969. The path analysis of categorical data. ETS Research Bulletin Series 2: i-15.
Wetzel, R. G., 1975. Limnology. Saunders, Philadelphia.
Wetzel, R. G. & G. E. Likens, 2000. Limnological Analyses, 3rd ed. Springer, New York.
Whitton, B. A., A. H. Al-Shehri, N. T. W. Ellwood & B. L. Turner, 2005. Ecological aspects of phosphatase activity in cyanobacteria, eukaryotic algae and bryophytes. In Turner, B. L., E. Frossard & D. S. Baldwin (eds), Organic Phosphorus in the Environment. Commonwealth Agricultural Bureau, Wallingford: 205–241.
Wright, S., 1934. The method of path coefficients. The Annals of Mathematical Statistics 5: 161–215.
Yue, S., P. Pilon, B. Phinney & G. Cavadias, 2002. The influence of autocorrelation on the ability to detect trend in hydrological series. Hydrological Processes 16: 1807–1829.
Acknowledgments
The data used in this study were collected as part of a research programme promoted by the International Commission for the Protection of Italian-Swiss Waters (CIPAIS). We gratefully acknowledge all the personnel that contributed to the monitoring program of Lake Lugano during the study period (1984–2018).
Funding
Open access funding provided by SUPSI - University of Applied Sciences and Arts of Southern Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Eric R. Larson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lepori, F., Capelli, C. Effects of phosphorus control on primary productivity and deep-water oxygenation: insights from Lake Lugano (Switzerland and Italy). Hydrobiologia 848, 613–629 (2021). https://doi.org/10.1007/s10750-020-04467-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04467-9